Bromocriptine Mesylate
Bromocriptine Mesylate Prescribing Information
Bromocriptine mesylate capsules are indicated for the treatment of dysfunctions associated with
Bromocriptine mesylate capsule therapy is indicated in the treatment of acromegaly. Bromocriptine mesylate capsule therapy, alone or as adjunctive therapy with pituitary irradiation or surgery, reduces serum growth hormone by 50% or more in approximately ½ of patients treated, although not usually to normal levels.
Since the effects of external pituitary radiation may not become maximal for several years, adjunctive therapy with bromocriptine mesylate capsule offers potential benefit before the effects of irradiation are manifested.
Bromocriptine mesylate capsules are indicated in the treatment of the signs and symptoms of idiopathic or postencephalitic Parkinson's disease. As adjunctive treatment to levodopa (alone or with a peripheral decarboxylase inhibitor), bromocriptine mesylate capsule therapy may provide additional therapeutic benefits in those patients who are currently maintained on optimal dosages of levodopa, those who are beginning to deteriorate (develop tolerance) to levodopa therapy, and those who are experiencing "end of dose failure" on levodopa therapy. Bromocriptine mesylate capsule therapy may permit a reduction of the maintenance dose of levodopa and, thus may ameliorate the occurrence and/or severity of adverse reactions associated with long-term levodopa therapy such as abnormal involuntary movements (e.g., dyskinesias) and the marked swings in motor function ("on-off" phenomenon). Continued efficacy of bromocriptine mesylate capsule therapy during treatment of more than 2 years has not been established.
Data are insufficient to evaluate potential benefit from treating newly diagnosed Parkinson's disease with bromocriptine mesylate capsules. Studies have shown, however, significantly more adverse reactions (notably nausea, hallucinations, confusion and hypotension) in bromocriptine-treated patients than in levodopa/carbidopa-treated patients. Patients unresponsive to levodopa are poor candidates for bromocriptine mesylate capsule therapy.
It is recommended that bromocriptine mesylate capsules be taken with food. Patients should be evaluated frequently during dose escalation to determine the lowest dosage that produces a therapeutic response.
The initial dosage of bromocriptine mesylate tablets in adults is one ½ to one 2½ mg scored tablet daily. An additional 2½ mg tablet may be added to the treatment regimen as tolerated every 2 to 7 days until an optimal therapeutic response is achieved. The therapeutic dosage ranged from 2.5 to 15 mg daily in adults studied clinically.
Based on limited data in children of age 11 to 15,
In order to reduce the likelihood of prolonged exposure to bromocriptine mesylate capsules should an unsuspected pregnancy occur, a mechanical contraceptive should be used in conjunction with bromocriptine mesylate capsule therapy until normal ovulatory menstrual cycles have been restored. Contraception may then be discontinued in patients desiring pregnancy.
Thereafter, if menstruation does not occur within 3 days of the expected date, bromocriptine mesylate capsule therapy should be discontinued and a pregnancy test performed.
Virtually all acromegalic patients receiving therapeutic benefit from bromocriptine mesylate capsules also have reductions in circulating levels of growth hormone. Therefore, periodic assessment of circulating levels of growth hormone will, in most cases, serve as a guide in determining the therapeutic potential of bromocriptine. If, after a brief trial with bromocriptine mesylate capsule therapy, no significant reduction in growth hormone levels has taken place, careful assessment of the clinical features of the disease should be made, and if no change has occurred, dosage adjustment or discontinuation of therapy should be considered.
The initial recommended dosage is one ½ to one 2½ mg bromocriptine mesylate tablet on retiring (with food) for 3 days. An additional one ½ to 1 bromocriptine mesylate tablet should be added to the treatment regimen as tolerated every 3 to 7 days until the patient obtains optimal therapeutic benefit. Patients should be reevaluated monthly and the dosage adjusted based on reductions of growth hormone or clinical response. The usual optimal therapeutic dosage range of bromocriptine mesylate capsules varies from 20 mg/day to 30 mg/day in most patients. The maximal dosage should not exceed 100 mg/day.
Patients treated with pituitary irradiation should be withdrawn from bromocriptine mesylate capsule therapy on a yearly basis to assess both the clinical effects of radiation on the disease process as well as the effects of bromocriptine mesylate capsule therapy. Usually a 4 week to 8 week withdrawal period is adequate for this purpose. Recurrence of the signs/symptoms or increases in growth hormone indicate the disease process is still active and further courses of bromocriptine should be considered.
The basic principle of bromocriptine mesylate capsule therapy is to initiate treatment at a low dosage and, on an individual basis, increase the daily dosage slowly until a maximum therapeutic response is achieved. The dosage of levodopa during this introductory period should be maintained, if possible. The initial dose of bromocriptine is one ½ of a 2½ mg tablet twice daily with meals. Assessments are advised at 2-week intervals during dosage titration to ensure that the lowest dosage producing an optimal therapeutic response is not exceeded. If necessary, the dosage may be increased every 14 to 28 days by 2½ mg/day with meals. Should it be advisable to reduce the dosage of levodopa because of adverse reactions, the daily dosage of bromocriptine, if increased, should be accomplished gradually in small (2½ mg) increments.
The safety of bromocriptine mesylate capsules have not been demonstrated in dosages exceeding 100 mg/day.
Hypersensitivity to bromocriptine or to any of the excipients of bromocriptine mesylate capsules, uncontrolled hypertension and sensitivity to any ergot alkaloids. In patients being treated for hyperprolactinemia, bromocriptine mesylate capsules should be withdrawn when pregnancy is diagnosed
The drug should not be used during the postpartum period in women with a history of coronary artery disease and other severe cardiovascular conditions unless withdrawal is considered medically contraindicated. If the drug is used in the postpartum period, the patient should be observed with caution.
The incidence of adverse effects is quite high (69%) but these are generally mild to moderate in degree. Therapy was discontinued in approximately 5% of patients because of adverse effects. These in decreasing order of frequency are: nausea (49%), headache (19%), dizziness (17%), fatigue (7%), lightheadedness (5%), vomiting (5%), abdominal cramps (4%), nasal congestion (3%), constipation (3%), diarrhea (3%) and drowsiness (3%).
A slight hypotensive effect may accompany bromocriptine mesylate treatment. The occurrence of adverse reactions may be lessened by temporarily reducing dosage to ½ a bromocriptine mesylate tablet 2 or 3 times daily. A few cases of cerebrospinal fluid rhinorrhea have been reported in patients receiving bromocriptine for treatment of large prolactinomas. This has occurred rarely, usually only in patients who have received previous transsphenoidal surgery, pituitary radiation, or both, and who were receiving bromocriptine for tumor recurrence. It may also occur in previously untreated patients whose tumor extends into the sphenoid sinus.
The most frequent adverse reactions encountered in acromegalic patients treated with bromocriptine were: nausea (18%), constipation (14%), postural/orthostatic hypotension (6%), anorexia (4%), dry mouth/nasal stuffiness (4%), indigestion/dyspepsia (4%), digital vasospasm (3%), drowsiness/tiredness (3%) and vomiting (2%).
Less frequent adverse reactions (less than 2%) were: gastrointestinal bleeding, dizziness, exacerbation of Raynaud's syndrome, headache and syncope. Rarely (less than 1%) hair loss, alcohol potentiation, faintness, lightheadedness, arrhythmia, ventricular tachycardia, decreased sleep requirement, visual hallucinations, lassitude, shortness of breath, bradycardia, vertigo, paresthesia, sluggishness, vasovagal attack, delusional psychosis, paranoia, insomnia, heavy headedness, reduced tolerance to cold, tingling of ears, facial pallor and muscle cramps have been reported.
In clinical trials in which bromocriptine was administered with concomitant reduction in the dose of levodopa/carbidopa, the most common newly appearing adverse reactions were: nausea, abnormal involuntary movements, hallucinations, confusion, "on-off" phenomenon, dizziness, drowsiness, faintness/fainting, vomiting, asthenia, abdominal discomfort, visual disturbance, ataxia, insomnia, depression, hypotension, shortness of breath, constipation, and vertigo.
Less common adverse reactions which may be encountered include: anorexia, anxiety, blepharospasm, dry mouth, dysphagia, edema of the feet and ankles, erythromelalgia, epileptiform seizure, fatigue, headache, lethargy, mottling of skin, nasal stuffiness, nervousness, nightmares, paresthesia, skin rash, urinary frequency, urinary incontinence, urinary retention, and rarely, signs and symptoms of ergotism such as tingling of fingers, cold feet, numbness, muscle cramps of feet and legs or exacerbation of Raynaud's syndrome.
Abnormalities in laboratory tests may include elevations in blood urea nitrogen, SGOT, SGPT, GGPT, CPK, alkaline phosphatase and uric acid, which are usually transient and not of clinical significance.
The following adverse reactions have been reported during postapproval use of bromocriptine (All Indications Combined). Because adverse reactions from spontaneous reports are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Confusion, psychomotor agitation/excitation, hallucinations, psychotic disorders, insomnia, libido increase, hypersexuality and impulse control/compulsive behaviors (including gambling, spending, and other intense urges).
Headache, drowsiness, dizziness, dyskinaesia, somnolence, paraesthesia, excess daytime somnolence, sudden onset of sleep.
Visual disturbance, vision blurred.
Tinnitus.
Pericardial effusion, constrictive pericarditis, tachycardia, bradycardia, arrhythmia, cardiac valve fibrosis.
Hypotension, orthostatic hypotension (very rarely leading to syncope), reversible pallor of fingers and toes induced by cold (especially in patients with history of Raynaud's phenomenon).
Nasal congestion, pleural effusion, pleural fibrosis, pleurisy, pulmonary fibrosis, dyspnoea.
Nausea, constipation, vomiting, dry mouth, diarrhea, abdominal pain, retroperitoneal fibrosis, gastrointestinal ulcer, gastrointestinal hemorrhage.
Allergic skin reactions, hair loss.
Leg cramps.
Fatigue, peripheral oedema, a syndrome resembling Neuroleptic Malignant Syndrome on abrupt withdrawal of bromocriptine, withdrawal symptoms (including apathy, anxiety, depression, fatigue, insomnia, sweating, and pain) with taper or after discontinuation
In postpartum studies with bromocriptine 23 percent of postpartum patients treated had at least 1 side effect, but they were generally mild to moderate in degree. Therapy was discontinued in approximately 3% of patients. The most frequently occurring adverse reactions were: headache (10%), dizziness (8%), nausea (7%), vomiting (3%), fatigue (1.0%), syncope (0.7%), diarrhea (0.4%) and cramps (0.4%). Decreases in blood pressure (≥ 20 mm Hg systolic and ≥ 10 mm Hg diastolic) occurred in 28% of patients at least once during the first 3 postpartum days; these were usually of a transient nature. Reports of fainting in the puerperium may possibly be related to this effect. In postmarketing experience in the U.S., serious adverse reactions reported include 72 cases of seizures (including 4 cases of status epilepticus), 30 cases of stroke, and 9 cases of myocardial infarction among postpartum patients. Seizure cases were not necessarily accompanied by the development of hypertension. An unremitting and often progressively severe headache, sometimes accompanied by visual disturbance, often preceded by hours to days many cases of seizure and/or stroke. Most patients had shown no evidence of any of the hypertensive disorders of pregnancy including eclampsia, preeclampsia or pregnancy-induced hypertension. One stroke case was associated with sagittal sinus thrombosis, and another was associated with cerebral and cerebellar vasculitis. One case of myocardial infarction was associated with unexplained disseminated intravascular coagulation and a second occurred in conjunction with use of another ergot alkaloid. The relationship of these adverse reactions to bromocriptine administration has not been established.
In rare cases serious adverse events, including hypertension, myocardial infarction, seizures, stroke, or psychic disorders have been reported in postpartum women treated with bromocriptine. In some patients the development of seizures or stroke was preceded by severe headache and/or transient visual disturbances. Although the causal relationship of these events to the drug is uncertain, periodic monitoring of blood pressure is advisable in postpartum women receiving bromocriptine. If hypertension, severe, progressive, or unremitting headache (with or without visual disturbances), or evidence of CNS toxicity develop, the administration of bromocriptine should be discontinued and the patient should be evaluated promptly.
Particular caution is required in patients who have recently been treated or are on concomitant therapy with drugs that can alter blood pressure, e.g., vasoconstrictors such as sympathomimetics or ergot alkaloids including ergometrine or methylergometrine and their concomitant use in the puerperium is not recommended.
To report SUSPECTED ADVERSE REACTIONS, contact Zydus Pharmaceuticals (USA) Inc. at 1-877-993-8779 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Bromocriptine mesylate is an ergot derivative with potent dopamine receptor agonist activity. Bromocriptine mesylate is chemically designated as Ergotaman-3΄, 6΄, 18-trione, 2-bromo-12΄-hydroxy-2΄-(1-methylethyl)-5΄-(2-methylpropyl)-, (5΄α)-mono-methanesulfonate (salt).
The structural formula is:
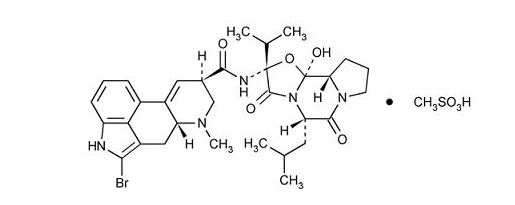
| C32H40BrN5O5.CH4SO3 | Mol. wt. 750.70 |
Bromocriptine mesylate, USP is white or slightly colored, fine crystalline powder and odorless or having a weak, characteristic odor.
Each bromocriptine mesylate capsule USP, 5 mg intended for oral administration contains bromocriptine mesylate equivalent to 5 mg of bromocriptine. In addition, each capsule contains the following inactive ingredients: carrageenan, colloidal silicon dioxide, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, maleic acid, potassium hydroxide and titanium dioxide. Each capsule is printed with black pharmaceutical ink and has following inactive ingredients: black iron oxide, potassium hydroxide, propylene glycol, purified water, shellac and strong ammonia solution.
Bromocriptine mesylate is a dopamine receptor agonist, which activates post-synaptic dopamine receptors. The dopaminergic neurons in the tuberoinfundibular process modulate the secretion of prolactin from the anterior pituitary by secreting a prolactin inhibitory factor (thought to be dopamine); in the corpus striatum the dopaminergic neurons are involved in the control of motor function. Clinically, bromocriptine significantly reduces plasma levels of prolactin in patients with physiologically elevated prolactin as well as in patients with hyperprolactinemia. The inhibition of physiological lactation as well as galactorrhea in pathological hyperprolactinemic states is obtained at dose levels that do not affect secretion of other tropic hormones from the anterior pituitary. Experiments have demonstrated that bromocriptine induces long-lasting stereotyped behavior in rodents and turning behavior in rats having unilateral lesions in the substantia nigra. These actions, characteristic of those produced by dopamine, are inhibited by dopamine antagonists and suggest a direct action of bromocriptine on striatal dopamine receptors.
Bromocriptine mesylate is a nonhormonal, nonestrogenic agent that inhibits the secretion of prolactin in humans, with little or no effect on other pituitary hormones, except in patients with acromegaly, where it lowers elevated blood levels of growth hormone in the majority of patients.
Bromocriptine mesylate produces its therapeutic effect in the treatment of Parkinson's disease, a clinical condition characterized by a progressive deficiency in dopamine synthesis in the substantia nigra, by directly stimulating the dopamine receptors in the corpus striatum. In contrast, levodopa exerts its therapeutic effect only after conversion to dopamine by the neurons of the substantia nigra, which are known to be numerically diminished in this patient population.