Bupivacaine Hydrochloride
Bupivacaine Hydrochloride Prescribing Information
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
Most of sections are re-formatted.
Bupivacaine Hydrochloride in Dextrose Injection, USP is indicated for subarachnoid injection in adults for the production of subarachnoid block (spinal anesthesia).
Bupivacaine Hydrochloride in Dextrose Injection, USP is a clear, colorless solution available as:
- 15 mg/2 mL (7.5 mg/mL) in single-dose glass ampules.
Bupivacaine Hydrochloride in Dextrose Injection, USP is contraindicated in:
- intravenous regional anesthesia (Bier Block) [see].
5.8 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride in Dextrose Injection, USP in this procedure is lacking. Therefore, Bupivacaine Hydrochloride in Dextrose Injection, USP is contraindicated for use with this technique [
see Contraindications (4)]. - patients with septicemia.
- patients with severe hemorrhage, severe hypotension or shock, due to a reduced cardiac output.
- patients with clinically significant arrhythmias, such as complete heartblock, due a reduced cardiac output.
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride in Dextrose Injection, USP.
- patients with local infection at the site of proposed lumbar puncture.
The following clinically significant adverse reactions have been reported and described in other sections of the labeling:
- Allergic-Type Reactions [see]
4 CONTRAINDICATIONSBupivacaine Hydrochloride in Dextrose Injection, USP is contraindicated in:
- intravenous regional anesthesia (Bier Block) [see Warnings and Precautions (5.8)].
- patients with septicemia.
- patients with severe hemorrhage, severe hypotension or shock, due to a reduced cardiac output.
- patients with clinically significant arrhythmias, such as complete heartblock, due a reduced cardiac output.
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride in Dextrose Injection, USP.
- patients with local infection at the site of proposed lumbar puncture.
- intravenous regional anesthesia (Bier Block) [
- Dose-Related Toxicity [see]
5.3 Dose-Related ToxicityThe safety and effectiveness of Bupivacaine Hydrochloride in Dextrose Injection, USP depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Careful and constant monitoring of cardiovascular and respiratory (adequacy of oxygenation and ventilation) vital signs and the patient’s state of consciousness should be performed after injection of Bupivacaine Hydrochloride in Dextrose Injection, USP solutions.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of dose-related toxicity, hypoventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and possibly death.
The patient should have an indwelling intravenous catheter to assure adequate intravenous access. Use the lowest dosage of Bupivacaine Hydrochloride in Dextrose Injection, USP that results in effective anesthesia to avoid serious adverse reactions. Avoid rapid injection of a large volume of Bupivacaine Hydrochloride in Dextrose Injection, USP.
Injection of repeated doses of Bupivacaine Hydrochloride in Dextrose Injection, USP may cause significant increases in plasma bupivacaine levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status. Reduced doses may be indicated in patients with increased intra-abdominal pressure (including obstetrical patients), if otherwise suitable for spinal anesthesia.
- Systemic Toxicities with Unintended Intravascular Injection [see]
5.4 Risk of Systemic Toxicities with Unintended Intravascular InjectionUnintended intravascular injection of Bupivacaine Hydrochloride in Dextrose Injection, USP may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest [
see Adverse Reactions (6)].Aspirate for blood and cerebrospinal fluid before injecting Bupivacaine Hydrochloride in Dextrose Injection, USP, for both the initial dose and all subsequent doses (where applicable), to confirm entry into the subarachnoid space and to avoid intravascular injection. Aspiration of cerebrospinal fluid into a Bupivacaine Hydrochloride in Dextrose Injection, USP-filled syringe will result in an identifiable swirl in the solution. A negative aspiration for blood does not ensure against an intravascular injection.
- Methemoglobinemia [see]
5.5 MethemoglobinemiaCases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose 6 phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition [
see Drug Interactions (7.2)]. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure, and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Bupivacaine Hydrochloride in Dextrose Injection, USP and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
- Cardiac Arrest in Obstetrical Anesthesia [see]
5.6 Risk of Cardiac Arrest with Use of Epidural Bupivacaine in Obstetrical AnesthesiaThere have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride Injection, USP for epidural anesthesia in obstetrical patients. In most cases, this has followed use of Bupivacaine Hydrochloride Injection, USP 0.75%, not Bupivacaine Hydrochloride in Dextrose Injection, USP. The package insert for Bupivacaine Hydrochloride Injection, USP for epidural, nerve block, etc., has a more complete discussion of preparation for, and management of cardiac arrest following epidural administration. Bupivacaine Hydrochloride in Dextrose Injection, USP is recommended for spinal anesthesia in obstetrical patients.
- Chondrolysis with Intra-Articular Infusion [see]
5.7 Chondrolysis with Intra-Articular InfusionIntra-articular infusions of local anesthetics including bupivacaine following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are not associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness, and loss of motion can be variable, but may begin as early as the 2ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
- Cardiac Arrest with Intravenous Regional Anesthesia Use [see],
4 CONTRAINDICATIONSBupivacaine Hydrochloride in Dextrose Injection, USP is contraindicated in:
- intravenous regional anesthesia (Bier Block) [see Warnings and Precautions (5.8)].
- patients with septicemia.
- patients with severe hemorrhage, severe hypotension or shock, due to a reduced cardiac output.
- patients with clinically significant arrhythmias, such as complete heartblock, due a reduced cardiac output.
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride in Dextrose Injection, USP.
- patients with local infection at the site of proposed lumbar puncture.
5.8 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride in Dextrose Injection, USP in this procedure is lacking. Therefore, Bupivacaine Hydrochloride in Dextrose Injection, USP is contraindicated for use with this technique [
see Contraindications (4)]. - intravenous regional anesthesia (Bier Block) [
The following adverse reactions from voluntary reports or clinical studies have been reported with bupivacaine. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions to Bupivacaine Hydrochloride in Dextrose Injection, USP are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to Bupivacaine Hydrochloride in Dextrose Injection, USP is due to cephalad extension of the motor level of anesthesia and/or excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse reactions that demand immediate counter-measures following the administration of spinal anesthesia were hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia. These have led to cardiac arrest if untreated. In addition, dose-related convulsions and cardiovascular collapse have resulted from diminished tolerance, rapid absorption from the injection site, or from unintentional intravascular injection of a local anesthetic solution.
Unintended intravascular injection of Bupivacaine Hydrochloride in Dextrose Injection, USP may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest [
Aspirate for blood and cerebrospinal fluid before injecting Bupivacaine Hydrochloride in Dextrose Injection, USP, for both the initial dose and all subsequent doses (where applicable), to confirm entry into the subarachnoid space and to avoid intravascular injection. Aspiration of cerebrospinal fluid into a Bupivacaine Hydrochloride in Dextrose Injection, USP-filled syringe will result in an identifiable swirl in the solution. A negative aspiration for blood does not ensure against an intravascular injection.
High doses or inadvertent intravascular injection have led to high plasma levels and related CNS toxicity. Adverse reactions were characterized by excitation and/or depression of the CNS and included restlessness, anxiety, dizziness, tinnitus, blurred vision, tremors, convulsions, drowsiness, unconsciousness, and respiratory arrest.
The incidences of adverse neurologic reactions associated with the use of local anesthetics may be related to the total dose of local anesthetic administered and are also dependent upon the particular drug used, the route of administration, and the physical status of the patient.
Neurologic effects following spinal anesthesia have included loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness and paralysis of the lower extremities, and loss of sphincter control with slow, incomplete, or no recovery; hypotension, high or total spinal block; urinary retention; headache; backache; septic meningitis, meningismus; arachnoiditis; slowing of labor; increased incidence of forceps delivery; shivering; cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid; and fecal and urinary incontinence.
Bupivacaine Hydrochloride in Dextrose Injection, USP is an amide-local anesthetic and sterile hyperbaric aqueous solution. The route of administration for Bupivacaine Hydrochloride in Dextrose Injection, USP is by subarachnoid injection. Bupivacaine Hydrochloride in Dextrose Injection, USP contains bupivacaine hydrochloride, as the active pharmaceutical ingredient and also contains Dextrose, as baricity agent.
Bupivacaine Hydrochloride (monohydrate) chemical name is 2-piperidinecarboxamide, 1-butyl-N-(2,6dimethylphenyl)-, monohydrochloride, monohydrate, a white crystalline powder that is freely soluble in 95 percent ethanol, soluble in water, and slightly soluble in chloroform or acetone. Bupivacaine Hydrochloride (monohydrate) has a molecular formula of C18H28N2O·HCl·H2O and molecular weight of 342.90 g/mol and has the following structural formula:
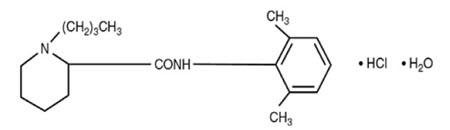
Dextrose chemical name is D-glucopyranose. Dextrose (anhydrous) has a molecular formula of C6H12O6, molecular weight of 180.16 g/mol and has the following structural formula:
-formula.jpg)
Bupivacaine Hydrochloride in Dextrose Injection, USP is a clear and colorless sterile hyperbaric solution.
Each mL of Bupivacaine Hydrochloride in Dextrose Injection, USP contains 7.5 mg bupivacaine hydrochloride (anhydrous) (equivalent to 7.9 mg of bupivacaine hydrochloride monohydrate), 82.5 mg dextrose (anhydrous) as baricity agent, and sodium hydroxide and hydrochloric acid as pH adjusters in water for injection.
Bupivacaine Hydrochloride in Dextrose Injection, USP pH is between 4.0 and 6.5.
The specific gravity of Bupivacaine Hydrochloride in Dextrose Injection, USP is between 1.030 and 1.035 at 25°C and 1.03 at 37°C.
Bupivacaine Hydrochloride in Dextrose Injection, USP does not contain any preservatives.