Bupivacaine Hydrochloride Prescribing Information
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary.
Bupivacaine Hydrochloride injection is indicated in adults for the production of local or regional anesthesia or analgesia for surgery, dental and oral surgery procedures, diagnostic and therapeutic procedures, and for obstetrical procedures. Specific concentrations and presentations of Bupivacaine Hydrochloride injection are recommended for each type of block indicated to produce local or regional anesthesia or analgesia
The dosage of Bupivacaine Hydrochloride injection administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. Administer the smallest dosage and concentration required to produce the desired result.
The types of block and recommended Bupivacaine Hydrochloride injection concentrations are shown in Table 1.
| Type of Block | Bupivacaine Hydrochloride injection | ||
|---|---|---|---|
| 0.25% (2.5 mg/mL) | 0.5% (5 mg/mL) | 0.75% (7.5 mg/mL)* | |
Local infiltration | ✓ | ||
Peripheral nerve block | ✓ | ✓ | |
Retrobulbar block | ✓ | ||
Sympathetic block | ✓ | ||
Caudal block | ✓ | ✓ | |
Lumbar epidural block | ✓ | ✓ | ✓ |
Epidural test dose | |||
Dental block | |||
* Bupivacaine Hydrochloride injection0.75% (7.5 mg/mL) is not recommended for nonobstetrical surgical procedures in pregnant patients.
✓= indicated use
At recommended dosages, Bupivacaine Hydrochloride injection produces complete sensory block, but the effect on motor function differs among the three concentrations. Table 2 provides information on the expected effect on motor function for the three concentrations.
Bupivacaine Hydrochloride injectionConcentration | Motor Function |
0.25% (2.5 mg/mL) | When used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of action may be slower than with the 0.5% (5 mg/mL) or 0.75% (7.5 mg/mL) solutions. |
0.5% (5 mg/mL) | Provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which complete muscle relaxation is essential. |
0.75% (7.5 mg/mL) | Produces complete motor block. Most useful for epidural block in abdominal operations requiring complete muscle relaxation, and for retrobulbar anesthesia. Not for obstetrical anesthesia. |
The duration of anesthesia with Bupivacaine Hydrochloride injection is such that for most indications, a single-dose is sufficient.
The maximum dosage limit within the recommended dosage range must be individualized in each case after evaluating the size and physical status of the patient, as well as the anticipated rate of systemic absorption from a particular injection site.
The dosages in Table 3 are recommended as a guide for use in the average adult. These doses may be repeated once every three hours. Do not exceed a total daily dosage of 400 mg in 24 hours. The duration of anesthetic effect may be prolonged by the addition of epinephrine.
Type of Block | Concentration of Bupivacaine Hydrochloride injection | Each Dose | Motor Blocka | |
mL | mg of Bupivacaine Hydrochloride injection | |||
Local infiltration | 0.25% (2.5 mg/mL)b | Up to 70 (without epinephrine) | Up to 175 (without epinephrine) | ― |
Peripheral nerve block | 0.5% (5 mg/mL)b | 5-35 (without epinephrine) | 25-175 (without epinephrine) | moderate to complete |
0.25% (2.5 mg/mL)b | 5-70 (without epinephrine) | 12.5-175 (without epinephrine) | moderate to complete | |
Retrobulbar block [see Dosage and Administration (2.6)] | 0.75% (7.5 mg/mL) | 2-4 | 15-30 | complete |
Sympathetic block | 0.25% (2.5 mg/mL) | 20-50 | 50-125 | ― |
Caudal block | 0.5% (5 mg/mL)b | 15-30 | 75-150 | moderate to complete |
0.25% (2.5 mg/mL)b | 15-30 | 37.5-75 | moderate | |
Lumbar epidural block [see Dosage and Administration (2.3)] | 0.75% (7.5 mg/mL)c | 10-20 | 75-150 | complete |
0.5% (5 mg/mL)b | 10-20 | 50-100 | moderate to complete | |
0.25% (2.5 mg/mL)b | 10-20 | 25-50 | partial to moderate | |
- With continuous (intermittent) techniques, repeat doses increase the degree of motor block. The first repeat dose of 0.5% (5 mg/mL) may produce complete motor block. Intercostal nerve block with 0.25% (2.5 mg/mL) also may produce complete motor block for intra-thoracic and upper intra-abdominal surgery.
- Solutions with or without epinephrine (i.e., applies to Bupivacaine Hydrochloride injection)
- For single-dose use; not for intermittent epidural technique. Not for obstetrical anesthesia.
Not all blocks are indicated for use with Bupivacaine Hydrochloride injection given clinically significant risks associated with use
The dosage of Bupivacaine Hydrochloride injection administered varies with the anesthetic procedure, the area to be anesthetized, the vascularity of the tissues, the number of neuronal segments to be blocked, the depth of anesthesia and degree of muscle relaxation required, the duration of anesthesia desired, individual tolerance, and the physical condition of the patient. Administer the smallest dosage and concentration required to produce the desired result.
The types of block and recommended Bupivacaine Hydrochloride injection concentrations are shown in Table 1.
| Type of Block | Bupivacaine Hydrochloride injection | ||
|---|---|---|---|
| 0.25% (2.5 mg/mL) | 0.5% (5 mg/mL) | 0.75% (7.5 mg/mL)* | |
Local infiltration | ✓ | ||
Peripheral nerve block | ✓ | ✓ | |
Retrobulbar block | ✓ | ||
Sympathetic block | ✓ | ||
Caudal block | ✓ | ✓ | |
Lumbar epidural block | ✓ | ✓ | ✓ |
Epidural test dose | |||
Dental block | |||
* Bupivacaine Hydrochloride injection0.75% (7.5 mg/mL) is not recommended for nonobstetrical surgical procedures in pregnant patients.
✓= indicated use
At recommended dosages, Bupivacaine Hydrochloride injection produces complete sensory block, but the effect on motor function differs among the three concentrations. Table 2 provides information on the expected effect on motor function for the three concentrations.
Bupivacaine Hydrochloride injectionConcentration | Motor Function |
0.25% (2.5 mg/mL) | When used for caudal, epidural, or peripheral nerve block, produces incomplete motor block. Should be used for operations in which muscle relaxation is not important, or when another means of providing muscle relaxation is used concurrently. Onset of action may be slower than with the 0.5% (5 mg/mL) or 0.75% (7.5 mg/mL) solutions. |
0.5% (5 mg/mL) | Provides motor blockade for caudal, epidural, or nerve block, but muscle relaxation may be inadequate for operations in which complete muscle relaxation is essential. |
0.75% (7.5 mg/mL) | Produces complete motor block. Most useful for epidural block in abdominal operations requiring complete muscle relaxation, and for retrobulbar anesthesia. Not for obstetrical anesthesia. |
The duration of anesthesia with Bupivacaine Hydrochloride injection is such that for most indications, a single-dose is sufficient.
The maximum dosage limit within the recommended dosage range must be individualized in each case after evaluating the size and physical status of the patient, as well as the anticipated rate of systemic absorption from a particular injection site.
The dosages in Table 3 are recommended as a guide for use in the average adult. These doses may be repeated once every three hours. Do not exceed a total daily dosage of 400 mg in 24 hours. The duration of anesthetic effect may be prolonged by the addition of epinephrine.
Type of Block | Concentration of Bupivacaine Hydrochloride injection | Each Dose | Motor Blocka | |
mL | mg of Bupivacaine Hydrochloride injection | |||
Local infiltration | 0.25% (2.5 mg/mL)b | Up to 70 (without epinephrine) | Up to 175 (without epinephrine) | ― |
Peripheral nerve block | 0.5% (5 mg/mL)b | 5-35 (without epinephrine) | 25-175 (without epinephrine) | moderate to complete |
0.25% (2.5 mg/mL)b | 5-70 (without epinephrine) | 12.5-175 (without epinephrine) | moderate to complete | |
Retrobulbar block [see Dosage and Administration (2.6)] | 0.75% (7.5 mg/mL) | 2-4 | 15-30 | complete |
Sympathetic block | 0.25% (2.5 mg/mL) | 20-50 | 50-125 | ― |
Caudal block | 0.5% (5 mg/mL)b | 15-30 | 75-150 | moderate to complete |
0.25% (2.5 mg/mL)b | 15-30 | 37.5-75 | moderate | |
Lumbar epidural block [see Dosage and Administration (2.3)] | 0.75% (7.5 mg/mL)c | 10-20 | 75-150 | complete |
0.5% (5 mg/mL)b | 10-20 | 50-100 | moderate to complete | |
0.25% (2.5 mg/mL)b | 10-20 | 25-50 | partial to moderate | |
- With continuous (intermittent) techniques, repeat doses increase the degree of motor block. The first repeat dose of 0.5% (5 mg/mL) may produce complete motor block. Intercostal nerve block with 0.25% (2.5 mg/mL) also may produce complete motor block for intra-thoracic and upper intra-abdominal surgery.
- Solutions with or without epinephrine (i.e., applies to Bupivacaine Hydrochloride injection)
- For single-dose use; not for intermittent epidural technique. Not for obstetrical anesthesia.
Bupivacaine Hydrochloride injection is contraindicated in:
- obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- intravenous regional anesthesia (Bier Block)[see Warnings and Precautions (5.7)].
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride injection.
- Obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- Intravenous regional anesthesia (Bier Block).
- Known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride injection.
- Dose-Related Toxicity: Monitor cardiovascular and respiratory vital signs and patient’s state of consciousness after injection of Bupivacaine Hydrochloride injection.
- Methemoglobinemia: Cases of methemoglobinemia have been reported in association with local anesthetic use. See full prescribing information for more detail on managing these risks.
- Chondrolysis with Intra-Articular Infusion: Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions.
- Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block): There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block).
- Risk of Systemic Toxicities with Unintended Intravascular or Intrathecal Injection: Unintended intravascular or intrathecal injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Aspirate for blood or cerebrospinal fluid (where applicable) prior to each dose
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary.
The safety and effectiveness of Bupivacaine Hydrochloride injection depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be performed after injection of Bupivacaine Hydrochloride injection solutions.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of dose-related toxicity, underventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death.
During major regional nerve blocks, such as those of the brachial plexus or lower extremity, the patient should have an indwelling intravenous catheter to assure adequate intravenous access. Use the lowest dosage of Bupivacaine Hydrochloride injection that results in effective anesthesia to avoid high plasma levels and serious adverse effects. Avoid rapid injection of a large volume of Bupivacaine Hydrochloride solution and administer fractional (incremental) doses when feasible.
Injection of repeated doses of Bupivacaine Hydrochloride injection may cause significant increases in plasma levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status.
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition
Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Bupivacaine Hydrochloride injection and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride injection in this procedure is lacking. Therefore, Bupivacaine Hydrochloride injection is contraindicated for use with this technique
Unintended intravascular or intrathecal injection of Bupivacaine Hydrochloride injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Unintentional intrathecal injection during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column has resulted in underventilation or apnea (“Total or High Spinal”). A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia
Aspirate for blood or cerebrospinal fluid (where applicable) before injecting Bupivacaine Hydrochloride injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection.
Because amide local anesthetics such as bupivacaine are metabolized by the liver, consider reduced dosing and increased monitoring for bupivacaine systemic toxicity in patients with moderate to severe hepatic impairment who are treated with Bupivacaine Hydrochloride injection, especially with repeat doses
Bupivacaine Hydrochloride injection should be given in reduced doses in patients with impaired cardiovascular function (e.g., hypotension, heartblock) because they may be less able to compensate for functional changes associated with the prolongation of AV conduction produced by Bupivacaine Hydrochloride injection. Monitor patients closely for blood pressure, heart rate, and ECG changes.
Small doses of local anesthetics (e.g., Bupivacaine Hydrochloride injection) injected into the head and neck area, including retrobulbar, dental, and stellate ganglion blocks, may produce adverse reactions similar to systemic toxicity seen with unintentional intravascular injections of larger doses. The injection procedures require the utmost care.
Confusion, convulsions, respiratory depression, and/or respiratory arrest, and cardiovascular stimulation or depression have been reported. These reactions may be due to intra-arterial injection of the local anesthetic with retrograde flow to the cerebral circulation. They may also be due to puncture of the dural sheath of the optic nerve during retrobulbar block with diffusion of any local anesthetic along the subdural space to the midbrain. Monitor circulation and respiration and constantly observe patients receiving Bupivacaine Hydrochloride injection blocks. Resuscitative equipment and drugs, and personnel for treating adverse reactions should be immediately available. Dosage recommendations should not be exceeded
Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic injection. Prior to retrobulbar block (e.g., with Bupivacaine Hydrochloride injection), as with all other regional procedures, resuscitative equipment and drugs, and personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be immediately available
A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any other peripheral nerve block, including the facial nerve, and not indicated for local infiltration, including the conjunctiva
Because of the long duration of anesthesia, when Bupivacaine Hydrochloride injection with epinephrine [0.5% (5 mg/mL) of bupivacaine] is used for dental injections, warn patients about the possibility of inadvertent trauma to tongue, lips, and buccal mucosa and advise them not to chew solid foods until sensation returns
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary.
Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride injection in this procedure is lacking. Therefore, Bupivacaine Hydrochloride injection is contraindicated for use with this technique
Unintended intravascular or intrathecal injection of Bupivacaine Hydrochloride injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Unintentional intrathecal injection during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column has resulted in underventilation or apnea (“Total or High Spinal”). A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia
Aspirate for blood or cerebrospinal fluid (where applicable) before injecting Bupivacaine Hydrochloride injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection.
Bupivacaine hydrochloride injection, USP is a clear, colorless solution available as:
- 0.25% (25 mg/10 mL) (2.5 mg/mL) in single-dose vials.
- 0.25% (75 mg/30 mL) (2.5 mg/mL) in single-dose vials.
- 0.5% (50 mg/10 mL) (5 mg/mL) in single-dose vials.
- 0.5% (150 mg/30 mL) (5 mg/mL) in single-dose vials.
- 0.75% (75 mg/10 mL) (7.5 mg/mL) in single-dose vials.
- 0.75% (225 mg/30 mL) (7.5 mg/mL) in single-dose vials.
Bupivacaine Hydrochloride injection is contraindicated in:
- obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- intravenous regional anesthesia (Bier Block) [see.]5.7 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)
There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride injection in this procedure is lacking. Therefore, Bupivacaine Hydrochloride injection is contraindicated for use with this technique
[see Contraindications (4)]. - patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride injection.
The following clinically significant adverse reactions have been reported and described in the Warnings and Precautions section of the labeling:
- Cardiac Arrest in Obstetrical Anesthesia [see]5.1 Risk of Cardiac Arrest with Use of Bupivacaine Hydrochloride injection in Obstetrical Anesthesia
There have been reports of cardiac arrest with difficult resuscitation or death during use of Bupivacaine Hydrochloride injection for epidural anesthesia in obstetrical patients. In most cases, this has followed use of the 0.75% (7.5 mg/mL) concentration. Resuscitation has been difficult or impossible despite apparently adequate preparation and appropriate management. Cardiac arrest has occurred after convulsions resulting from systemic toxicity, presumably following unintentional intravascular injection. The 0.75% (7.5 mg/mL) concentration of Bupivacaine Hydrochloride injection is not recommended for obstetrical anesthesia and should be reserved for surgical procedures where a high degree of muscle relaxation and prolonged effect are necessary.
- Dose-Related Toxicity [see]5.2 Dose-Related Toxicity
The safety and effectiveness of Bupivacaine Hydrochloride injection depend on proper dosage, correct technique, adequate precautions, and readiness for emergencies. Careful and constant monitoring of cardiovascular and respiratory (adequacy of ventilation) vital signs and the patient’s state of consciousness should be performed after injection of Bupivacaine Hydrochloride injection solutions.
Possible early warning signs of central nervous system (CNS) toxicity are restlessness, anxiety, incoherent speech, lightheadedness, numbness and tingling of the mouth and lips, metallic taste, tinnitus, dizziness, blurred vision, tremors, twitching, CNS depression, or drowsiness. Delay in proper management of dose-related toxicity, underventilation from any cause, and/or altered sensitivity may lead to the development of acidosis, cardiac arrest, and, possibly, death.
During major regional nerve blocks, such as those of the brachial plexus or lower extremity, the patient should have an indwelling intravenous catheter to assure adequate intravenous access. Use the lowest dosage of Bupivacaine Hydrochloride injection that results in effective anesthesia to avoid high plasma levels and serious adverse effects. Avoid rapid injection of a large volume of Bupivacaine Hydrochloride solution and administer fractional (incremental) doses when feasible.
Injection of repeated doses of Bupivacaine Hydrochloride injection may cause significant increases in plasma levels with each repeated dose due to slow accumulation of the drug or its metabolites, or to slow metabolic degradation. Tolerance to elevated blood levels varies with the status of the patient. Debilitated, elderly patients and acutely ill patients should be given reduced doses commensurate with their age and physical status.
- Methemoglobinemia [see]5.3 Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition
[see Drug Interactions (7.5)]. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.Signs of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and/or abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious CNS and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death. Discontinue Bupivacaine Hydrochloride injection and any other oxidizing agents. Depending on the severity of the signs and symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. A more severe clinical presentation may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
- Chondrolysis with Intra-Articular Infusion [see]5.5 Chondrolysis with Intra-Articular Infusion
Intra-articular infusions of local anesthetics including Bupivacaine Hydrochloride injection following arthroscopic and other surgical procedures is an unapproved use, and there have been post-marketing reports of chondrolysis in patients receiving such infusions. The majority of reported cases of chondrolysis have involved the shoulder joint; cases of gleno-humeral chondrolysis have been described in pediatric and adult patients following intra-articular infusions of local anesthetics with and without epinephrine for periods of 48 to 72 hours. There is insufficient information to determine whether shorter infusion periods are associated with chondrolysis. The time of onset of symptoms, such as joint pain, stiffness and loss of motion can be variable, but may begin as early as the 2ndmonth after surgery. Currently, there is no effective treatment for chondrolysis; patients who experienced chondrolysis have required additional diagnostic and therapeutic procedures and some required arthroplasty or shoulder replacement.
- Cardiac Arrest with Intravenous Regional Anesthesia Use [see,4 CONTRAINDICATIONS
Bupivacaine Hydrochloride injection is contraindicated in:
- obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- intravenous regional anesthesia (Bier Block)[see Warnings and Precautions (5.7)].
- patients with a known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride injection.
- Obstetrical paracervical block anesthesia. Its use in this technique has resulted in fetal bradycardia and death.
- Intravenous regional anesthesia (Bier Block).
- Known hypersensitivity to bupivacaine or to any local anesthetic agent of the amide-type or to other components of Bupivacaine Hydrochloride injection.
]5.7 Risk of Cardiac Arrest with Intravenous Regional Anesthesia Use (Bier Block)There have been reports of cardiac arrest and death during the use of bupivacaine for intravenous regional anesthesia (Bier Block). Information on safe dosages and techniques of administration of Bupivacaine Hydrochloride injection in this procedure is lacking. Therefore, Bupivacaine Hydrochloride injection is contraindicated for use with this technique
[see Contraindications (4)]. - Systemic Toxicities with Unintended Intravascular or Intrathecal Injection [see]5.9 Risk of Systemic Toxicities with Unintended Intravascular or Intrathecal Injection
Unintended intravascular or intrathecal injection of Bupivacaine Hydrochloride injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Unintentional intrathecal injection during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column has resulted in underventilation or apnea (“Total or High Spinal”). A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia
[see Adverse Reactions (6)].Aspirate for blood or cerebrospinal fluid (where applicable) before injecting Bupivacaine Hydrochloride injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection.
- Respiratory Arrest Following Retrobulbar Block [see]5.15 Risk of Respiratory Arrest with Use in Ophthalmic Surgery
Clinicians who perform retrobulbar blocks should be aware that there have been reports of respiratory arrest following local anesthetic injection. Prior to retrobulbar block (e.g., with Bupivacaine Hydrochloride injection), as with all other regional procedures, resuscitative equipment and drugs, and personnel to manage respiratory arrest or depression, convulsions, and cardiac stimulation or depression should be immediately available
[see Warnings and Precautions (5.14)]. As with other anesthetic procedures, patients should be constantly monitored following ophthalmic blocks for signs of these adverse reactions, which may occur following relatively low total doses.A concentration of 0.75% bupivacaine is indicated for retrobulbar block; however, this concentration is not indicated for any other peripheral nerve block, including the facial nerve, and not indicated for local infiltration, including the conjunctiva
[see Indications and Usage (1)].
The following adverse reactions from voluntary reports or clinical studies have been reported with bupivacaine or bupivacaine and epinephrine. Because many of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions to Bupivacaine Hydrochloride injection are characteristic of those associated with other amide-type local anesthetics. A major cause of adverse reactions to this group of drugs is excessive plasma levels, which may be due to overdosage, unintentional intravascular injection, or slow metabolic degradation.
The most commonly encountered acute adverse reactions that demand immediate counter-measures were related to the CNS and the cardiovascular system. These adverse reactions were generally dose-related and due to high plasma levels which may have resulted from overdosage, rapid absorption from the injection site, diminished tolerance, or from unintentional intravascular injection of the local anesthetic solution. In addition to systemic dose-related toxicity, unintentional intrathecal injection of drug during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column (especially in the head and neck region) has resulted in underventilation or apnea (“Total or High Spinal”). Also, hypotension due to loss of sympathetic tone and respiratory paralysis or underventilation due to cephalad extension of the motor level of anesthesia have occurred. This has led to secondary cardiac arrest when untreated.
In the practice of caudal or lumbar epidural block, unintentional penetration of the subarachnoid space by the catheter or needle has occurred. Subsequent adverse effects may have depended partially on the amount of drug administered intrathecally and the physiological and physical effects of a dural puncture. A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia.
Neurologic effects following epidural or caudal anesthesia have included spinal block of varying magnitude (including high or total spinal block); hypotension secondary to spinal block; urinary retention; fecal and urinary incontinence; loss of perineal sensation and sexual function; persistent anesthesia, paresthesia, weakness, paralysis of the lower extremities and loss of sphincter control, all of which had slow, incomplete, or no recovery; headache; backache; septic meningitis; meningismus; slowing of labor; increased incidence of forceps delivery; and cranial nerve palsies due to traction on nerves from loss of cerebrospinal fluid.
Neurologic effects following other procedures or routes of administration have included persistent anesthesia, paresthesia, weakness, paralysis, all with slow, incomplete, or no recovery.
Unintended intravascular or intrathecal injection of Bupivacaine Hydrochloride injection may be associated with systemic toxicities, including CNS or cardiorespiratory depression and coma, progressing ultimately to respiratory arrest. Unintentional intrathecal injection during the intended performance of caudal or lumbar epidural block or nerve blocks near the vertebral column has resulted in underventilation or apnea (“Total or High Spinal”). A high spinal has been characterized by paralysis of the legs, loss of consciousness, respiratory paralysis, and bradycardia
Aspirate for blood or cerebrospinal fluid (where applicable) before injecting Bupivacaine Hydrochloride injection, both the initial dose and all subsequent doses, to avoid intravascular or intrathecal injection. However, a negative aspiration for blood or cerebrospinal fluid does not ensure against an intravascular or intrathecal injection.
Bupivacaine hydrochloride injection, USP contains bupivacaine hydrochloride, an amide local anesthetic, as the active pharmaceutical ingredient. The route of administration for Bupivacaine Hydrochloride injection is by injection, for infiltration, perineural, caudal, epidural, or retrobulbar use.
Bupivacaine hydrochloride is 2-piperidinecarboxamide, 1-butyl-
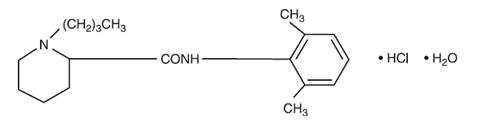
Bupivacaine hydrochloride injection, USP is a clear and colorless sterile isotonic solution. Each mL of single-dose vial contains 2.5 mg, 5 mg or 7.5 mg of bupivacaine hydrochloride (equivalent to 2.22 mg, 4.44 mg or 6.66 mg of bupivacaine, respectively), sodium chloride for isotonicity, sodium hydroxide or hydrochloric acid to adjust the pH between 4 and 6.5, in water for injection.