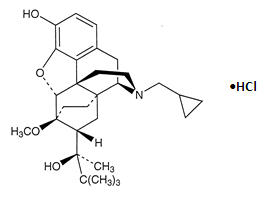
Buprenorphine Hydrochloride
Buprenorphine Hydrochloride Prescribing Information
Buprenorphine hydrochloride injection is indicated for the management of pain severe enough to require an opioid analgesic and for which alternate treatments are inadequate.
• Buprenorphine hydrochloride should be prescribed only by healthcare professionals who are knowledgeable about the use of opioids and how to mitigate the associated risks.• Use the lowest effective dosage for the shortest duration of time consistent with individual patient treatment goals [see]. Because the risk of overdose increases as opioid doses increase, reserve titration to higher doses of buprenorphine hydrochloride for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks.WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Many acute pain conditions (e.g., the pain that occurs with a number of surgical procedures or acute musculoskeletal injuries) require no more than a few days of an opioid analgesic. Clinical guidelines on opioid prescribing for some acute pain conditions are available.• There is variability in the opioid analgesic dose and duration needed to adequately manage pain due both to the cause of pain and to individual patient factors. Initiate the dosing regimen for each patient individually, taking into account the patient’s underlying cause and severity of pain, prior analgesic treatment and response, and risk factors for addiction, abuse, and misuse [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with buprenorphine hydrochloride. Consider this risk when selecting an initial dose and when making dose adjustments [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Inspect buprenorphine hydrochloride injection for particulate matter and discoloration prior to administration.
Buprenorphine hydrochloride is contraindicated in patients with:
• Significant respiratory depression [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Known or suspected gastrointestinal obstruction, including paralytic ileus [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].• Hypersensitivity to buprenorphine (e.g., anaphylaxis) or any other ingredient in buprenorphine hydrochloride [see].WARNINGSAddiction, Abuse, and MisuseBuprenorphine hydrochloride contains buprenorphine, a Schedule III controlled substance. As an opioid, buprenorphine hydrochloride exposes users to the risks of addiction, abuse, and misuse.
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed buprenorphine hydrochloride. Addiction can occur at recommended doses and if the drug is misused or abused.
Assess each patient's risk for opioid addiction, abuse, or misuse prior to prescribing buprenorphine hydrochloride, and reassess all patients receiving buprenorphine hydrochloride for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol addiction or abuse) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the prescribing of buprenorphine hydrochloride for the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as buprenorphine hydrochloride, but use in such patients necessitates intensive counseling about the risks and proper use of buprenorphine hydrochloride along with frequent reevaluation for signs of addiction, abuse, and misuse.
Opioids are sought for nonmedical use and are subject to diversion from legitimate prescribed use. Consider these risks when prescribing or dispensing buprenorphine hydrochloride. Strategies to reduce these risks include proper product storage and control practices for a C-III drug.
Contact local state professional licensing board or state controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Life-Threatening Respiratory DepressionSerious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory depression and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient's clinical status. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of buprenorphine hydrochloride, the risk is greatest during the initiation of therapy or following a dosage increase.
To reduce the risk of respiratory depression, proper dosing and titration of buprenorphine hydrochloride are essential. Overestimating the buprenorphine hydrochloride dosage when converting patients from another opioid product can result in a fatal overdose with the first dose.
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see
DOSAGE AND ADMINISTRATION].Risks from Concomitant Use with Benzodiazepines or Other CNS DepressantsProfound sedation, respiratory depression, coma, and death may result from the concomitant use of buprenorphine hydrochloride with benzodiazepines and/or other CNS depressants, including alcohol (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see
Drug Interactions].If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Monitor patients closely for signs and symptoms of respiratory depression and sedation.
Neonatal Opioid Withdrawal SyndromeUse of buprenorphine hydrochloride for an extended period of time during pregnancy can result in withdrawal in the neonate. Neonatal opioid withdrawal syndrome, unlike opioid withdrawal syndrome in adults, may be life-threatening if not recognized and treated, and requires management according to protocols developed by neonatology experts. Observe newborns for signs of neonatal opioid withdrawal syndrome and manage accordingly. Advise pregnant women using opioids for an extended period of time of the risk of neonatal opioid withdrawal syndrome and ensure that management by neonatology experts will be available at delivery [see
WARNINGS, PRECAUTIONS: Information for Patients, Pregnancy].Opioid-Induced Hyperalgesia and AllodyniaOpioid-Induced Hyperalgesia (OIH) occurs when an opioid analgesic paradoxically causes an increase in pain, or an increase in sensitivity to pain. This condition differs from tolerance, which is the need for increasing doses of opioids to maintain a defined effect [see
DEPENDENCE]. Symptoms of OIH include (but may not be limited to) increased levels of pain upon opioid dosage increase, decreased levels of pain upon opioid dosage decrease, or pain from ordinarily non-painful stimuli (allodynia). These symptoms may suggest OIH only if there is no evidence of underlying disease progression, opioid tolerance, opioid withdrawal, or addictive behavior.Cases of OIH have been reported, both with short-term and longer-term use of opioid analgesics. Though the mechanism of OIH is not fully understood, multiple biochemical pathways have been implicated. Medical literature suggests a strong biologic plausibility between opioid analgesics and OIH and allodynia. If a patient is suspected to be experiencing OIH, carefully consider appropriately decreasing the dose of the current opioid analgesic or opioid rotation (safely switching the patient to a different opioid moiety) [see
DOSAGE AND ADMINISTRATION, WARNINGS].Life-Threatening Respiratory Depression in Patients with Chronic Pulmonary Disease or in Elderly, Cachectic, or Debilitated PatientsThe use of buprenorphine hydrochloride in patients with acute or severe bronchial asthma in an unmonitored setting or in the absence of resuscitative equipment is contraindicated.
Patients with Chronic Pulmonary Disease:Buprenorphine hydrochloride-treated patients with significant chronic obstructive pulmonary disease or cor pulmonale, and those with substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression are at increased risk of decreased respiratory drive, including apnea, even at recommended dosages of buprenorphine hydrochloride [seeWARNINGS].Elderly, Cachectic, or Debilitated Patients:Life-threatening respiratory depression is more likely to occur in elderly, cachectic, or debilitated patients as they may have altered pharmacokinetics or altered clearance compared to younger, healthier patients.Monitor such patients closely, particularly when initiating and titrating buprenorphine hydrochloride and when buprenorphine hydrochloride is given concomitantly with other drugs that depress respiration [see
WARNINGS].Alternatively, consider the use of non-opioid analgesics in these patients.Adrenal InsufficiencyCases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use. Presentation of adrenal insufficiency may include non-specific symptoms and signs including nausea, vomiting, anorexia, fatigue, weakness, dizziness, and low blood pressure. If adrenal insufficiency is suspected, confirm the diagnosis with diagnostic testing as soon as possible. If adrenal insufficiency is diagnosed, treat with physiologic replacement doses of corticosteroids. Wean the patient off of the opioid to allow adrenal function to recover and continue corticosteroid treatment until adrenal function recovers. Other opioids may be tried as some cases reported use of a different opioid without recurrence of adrenal insufficiency. The information available does not identify any particular opioids as being more likely to be associated with adrenal insufficiency.
Severe HypotensionBuprenorphine hydrochloride may cause severe hypotension including orthostatic hypotension and syncope in ambulatory patients. There is increased risk in patients whose ability to maintain blood pressure has already been compromised by a reduced blood volume, or concurrent administration of certain CNS depressant drugs (e.g., phenothiazines or general anesthetics). Monitor these patients for signs of hypotension after initiating or titrating the dosage of buprenorphine hydrochloride. In patients with circulatory shock, buprenorphine hydrochloride may cause vasodilation that can further reduce cardiac output and blood pressure.
Avoid the use of buprenorphine hydrochloride in patients with circulatory shock.
Risks of Use in Patients with Increased Intracranial Pressure, Brain Tumors, Head Injury, or Impaired ConsciousnessIn patients who may be susceptible to the intracranial effects of CO2retention (e.g., those with evidence of increased intracranial pressure or brain tumors), buprenorphine hydrochloride may reduce respiratory drive, and the resultant CO2retention can further increase intracranial pressure. Monitor such patients for signs of sedation and respiratory depression, particularly when initiating therapy with buprenorphine hydrochloride.
Opioids may also obscure the clinical course in a patient with a head injury.
Avoid the use of buprenorphine hydrochloride in patients with impaired consciousness or coma.
QTc ProlongationThorough QT studies with buprenorphine products have demonstrated QT prolongation ≤ 15 msec. This QTc prolongation effect does not appear to be mediated by hERG channels. Based on these two findings, buprenorphine is unlikely to be pro‐arrhythmic when used alone in patients without risk factors. The risk of combining buprenorphine with other QT‐prolonging agents is not known.
Consider these observations in clinical decisions when prescribing buprenorphine hydrochloride to patients with risk factors such as hypokalemia, bradycardia, recent conversion from atrial fibrillation, congestive heart failure, digitalis therapy, baseline QT prolongation, subclinical long‐QT syndrome, or severe hypomagnesemia.
Anaphylactic/Allergic ReactionsCases of acute and chronic hypersensitivity to buprenorphine have been reported both in clinical trials and in post-marketing experience. The most common signs and symptoms include rashes, hives, and pruritus. Cases of bronchospasm, angioneurotic edema, and anaphylactic shock have been reported. Buprenorphine hydrochloride is contraindicated in patients with a history of hypersensitivity to buprenorphine.
Risks of Use in Patients with Gastrointestinal ConditionsBuprenorphine hydrochloride is contraindicated in patients with known or suspected gastrointestinal obstruction, including paralytic ileus.
The buprenorphine in buprenorphine hydrochloride may cause spasm of the sphincter of Oddi. Opioids may cause increases in the serum amylase. Monitor patients with biliary tract disease, including acute pancreatitis, for worsening symptoms.
Increased Risk of Seizures in Patients with Seizure DisordersThe buprenorphine in buprenorphine hydrochloride may increase the frequency of seizures in patients with seizure disorders, and may increase the risk of seizures in other clinical settings associated with seizures. Monitor patients with a history of seizure disorders for worsened seizure control during buprenorphine hydrochloride therapy.
Risks Driving and Operating MachineryBuprenorphine hydrochloride may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of buprenorphine hydrochloride and know how they will react to the medication [see
PRECAUTIONS: Information for Patients].
The most frequent side effect in clinical studies involving 1,133 patients was sedation which occurred in approximately two-thirds of the patients. Although sedated, these patients could easily be aroused to an alert state.
Nausea | Dizziness/Vertigo |
Sweating | Headache | |
Hypotension | Nausea/Vomiting | |
Vomiting | Hypoventilation | |
Miosis |
Other effects observed infrequently include malaise, hallucinations, depersonalization, coma, dyspepsia, flatulence, apnea, rash, amblyopia, tremor, and pallor.
The following reactions have been reported to occur rarely: loss of appetite, dysphoria/agitation, diarrhea, urticaria, and convulsions/lack of muscle coordination.
In the United Kingdom, buprenorphine hydrochloride was made available under monitored release regulation during the first year of sale, and yielded data from 1,736 physicians on 9,123 patients (17,120 administrations). Data on 240 children under the age of 18 years were included in this monitored release program. No important new adverse effects attributable to buprenorphine hydrochloride were observed.
Benzodiazepines and Other Central Nervous System (CNS) Depressants | |
Clinical Impact: | Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death. |
Intervention: | Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Inform patients and caregivers of this potential interaction and educate them on the signs and symptoms of respiratory depression (including sedation). |
Examples: | Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, and other opioids, alcohol. |
Inhibitors of CYP3A4 | |
Clinical Impact: | The concomitant use of buprenorphine and CYP3A4 inhibitors can increase the plasma concentration of buprenorphine, resulting in increased or prolonged opioid effects, particularly when an inhibitor is added after a stable dose of buprenorphine hydrochloride is achieved. After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the buprenorphine plasma concentration will decrease [see The limits of sensitivity of available analytical methodology precluded demonstration of bioequivalence between intramuscular and intravenous routes of administration. In postoperative adults, pharmacokinetic studies have shown elimination half-lives ranging from 1.2 to 7.2 hours (mean 2.2 hours) after intravenous administration of 0.3 mg of buprenorphine. A single, ten-patient, pharmacokinetic study of doses of 3μg/kg in children (age 5 to 7 years) showed a high inter-patient variability, but suggests that the clearance of the drug may be higher in children than in adults. This is supported by at least one repeat-dose study in postoperative pain that showed an optimal inter-dose interval of 4 to 5 hours in pediatric patients as opposed to the recommended 6 to 8 hours in adults. Buprenorphine undergoes both N-dealkylation to norbuprenorphine and glucuronidation. The N-dealkylation pathway is mediated primarily by CYP3A4. Norbuprenorphine, the major metabolite, can further undergo glucuronidation. Its clearance is related to hepatic blood flow. Studies in patients anesthetized with 0.5% halothane have shown that this anesthetic decreases hepatic blood flow by about 30%. |
Intervention: | If concomitant use is necessary, consider dosage reduction of buprenorphine hydrochloride until stable drug effects are achieved. Monitor patients for respiratory depression and sedation.
|
Examples: | Macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g., ketoconazole), protease inhibitors (e.g., ritonavir). |
CYP3A4 Inducers | |
Clinical Impact: | The concomitant use of buprenorphine and CYP3A4 inducers can decrease the plasma concentration of buprenorphine [see The limits of sensitivity of available analytical methodology precluded demonstration of bioequivalence between intramuscular and intravenous routes of administration. In postoperative adults, pharmacokinetic studies have shown elimination half-lives ranging from 1.2 to 7.2 hours (mean 2.2 hours) after intravenous administration of 0.3 mg of buprenorphine. A single, ten-patient, pharmacokinetic study of doses of 3μg/kg in children (age 5 to 7 years) showed a high inter-patient variability, but suggests that the clearance of the drug may be higher in children than in adults. This is supported by at least one repeat-dose study in postoperative pain that showed an optimal inter-dose interval of 4 to 5 hours in pediatric patients as opposed to the recommended 6 to 8 hours in adults. Buprenorphine undergoes both N-dealkylation to norbuprenorphine and glucuronidation. The N-dealkylation pathway is mediated primarily by CYP3A4. Norbuprenorphine, the major metabolite, can further undergo glucuronidation. Its clearance is related to hepatic blood flow. Studies in patients anesthetized with 0.5% halothane have shown that this anesthetic decreases hepatic blood flow by about 30%. After stopping a CYP3A4 inducer, as the effects of the inducer decline, the buprenorphine plasma concentration will increase [see The limits of sensitivity of available analytical methodology precluded demonstration of bioequivalence between intramuscular and intravenous routes of administration. In postoperative adults, pharmacokinetic studies have shown elimination half-lives ranging from 1.2 to 7.2 hours (mean 2.2 hours) after intravenous administration of 0.3 mg of buprenorphine. A single, ten-patient, pharmacokinetic study of doses of 3μg/kg in children (age 5 to 7 years) showed a high inter-patient variability, but suggests that the clearance of the drug may be higher in children than in adults. This is supported by at least one repeat-dose study in postoperative pain that showed an optimal inter-dose interval of 4 to 5 hours in pediatric patients as opposed to the recommended 6 to 8 hours in adults. Buprenorphine undergoes both N-dealkylation to norbuprenorphine and glucuronidation. The N-dealkylation pathway is mediated primarily by CYP3A4. Norbuprenorphine, the major metabolite, can further undergo glucuronidation. Its clearance is related to hepatic blood flow. Studies in patients anesthetized with 0.5% halothane have shown that this anesthetic decreases hepatic blood flow by about 30%. |
Intervention: | If concomitant use is necessary, consider increasing the buprenorphine hydrochloride dosage until stable drug effects are achieved. Monitor for signs of opioid withdrawal.
|
Examples: | Rifampin, carbamazepine, phenytoin |
Serotonergic Drugs | |
Clinical Impact: | The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system has resulted in serotonin syndrome. |
Intervention: | If concomitant use is warranted, carefully observe the patient, particularly during treatment initiation and dose adjustment. Discontinue buprenorphine hydrochloride if serotonin syndrome is suspected. |
Examples: | Selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue). |
Monoamine Oxidase Inhibitors (MAOIs) | |
Clinical Impact: | MAOI interactions with opioids may manifest as serotonin syndrome opioid toxicity (e.g., respiratory depression, coma). |
Intervention: | The use of buprenorphine hydrochloride is not recommended for patients taking MAOIs or within 14 days of stopping such treatment. |
Examples: | Phenelzine, tranylcypromine, linezolid |
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics | |
Clinical Impact: | May reduce the analgesic effect of buprenorphine hydrochloride and/or precipitate withdrawal symptoms. |
Intervention: | Avoid concomitant use. |
Examples: | Butorphanol, nalbuphine, pentazocine |
Muscle Relaxants | |
Clinical Impact: | Buprenorphine may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression. |
Intervention: | Monitor patients for signs of respiratory depression that may be greater than otherwise expected, decrease the dosage of buprenorphine hydrochloride and/or the muscle relaxant as necessary. |
Diuretics | |
Clinical Impact: | Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone. |
Intervention: | Monitor patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed. |
Anticholinergic Drugs | |
Clinical Impact: | The concomitant use of anticholinergic drugs may increase the risk of urinary retention and/or severe constipation, which may lead to paralytic ileus. |
Intervention: | Monitor patients for signs of urinary retention or reduced gastric motility when buprenorphine hydrochloride is used concomitantly with anticholinergic drugs. |
Antiretrovirals: Nucleoside reverse transcriptase inhibitors (NRTIs) | |
Clinical Impact: | Nucleoside reverse transcriptase inhibitors (NRTIs) do not appear to induce or inhibit the P450 enzyme pathway, thus no interactions with buprenorphine are expected. |
Intervention: | None |
Antiretrovirals: Non-nucleoside reverse transcriptase inhibitors (NNRTIs) | |
Clinical Impact: | Non-nucleoside reverse transcriptase inhibitors (NNRTIs) are metabolized principally by CYP3A4. Efavirenz, nevirapine, and etravirine are known CYP3A inducers, whereas delaviridine is a CYP3A inhibitor. Significant pharmacokinetic interactions between NNRTIs (e.g., efavirenz and delavirdine) and buprenorphine have been shown in clinical studies, but these pharmacokinetic interactions did not result in any significant pharmacodynamic effects. |
Intervention: | If prescribing an NNRTI to a patient taking buprenorphine hydrochloride, frequently reevaluate for this interaction and adjust dosing as necessary. |
Examples: | Efavirenz, nevirapine, etravirine, delavirdine |
Antiretrovirals: Protease inhibitors (PIs) | |
Clinical Impact: | Studies have shown some antiretroviral protease inhibitors (PIs) with CYP3A4 inhibitory activity (nelfinavir, lopinavir/ritonavir, ritonavir) have little effect on buprenorphine pharmacokinetic and no significant pharmacodynamic effects. Other PIs with CYP3A4 inhibitory activity (atazanavir and atazanavir/ritonavir) resulted in elevated levels of buprenorphine and norbuprenorphine, and patients in one study reported increased sedation. Symptoms of opioid excess have been found in post-marketing reports of patients receiving buprenorphine and atazanavir with and without ritonavir concomitantly. |
Intervention: | Monitor patients taking buprenorphine hydrochloride and atazanavir with and without ritonavir, and dose reduction of buprenorphine hydrochloride may be warranted. |
Examples: | Atazanavir, ritonavir |
Buprenorphine hydrochloride is a partial opioid agonist.
The chemical name of buprenorphine hydrochloride is 17-(cyclopropylmethyl)-α-(1,1-dimethylethyl)-4,5-epoxy 18,19-dihydro-3-hydroxy-6-methoxy-α-methyl-6,14-ethenomorphinan-7-methanol, hydrochloride [5α, 7α(S)].
Buprenorphine hydrochloride is a white powder, weakly acidic and with limited solubility in water.
Buprenorphine hydrochloride injection is a clear, sterile, injectable agonist-antagonist analgesic intended for intravenous or intramuscular administration. Each mL of buprenorphine hydrochloride injection contains 0.324 mg buprenorphine hydrochloride (equivalent to 0.3 mg buprenorphine), 50 mg anhydrous dextrose, water for injection and HCl to adjust pH to 3.5 to 5.5.
Buprenorphine hydrochloride has the molecular formula, C29H41NO4∙HCl and the molecular weight of 504.09. It has the following structural formula: