Bupropion Hydrochloride (Xl) Prescribing Information
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (Selective Serotonin Reuptake Inhibitors [SSRIs] and others) show that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in
Table 1: Risk Differences in the Number of Suicidality Cases by Age Group in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients | |
Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
Increases Compared to Placebo | |
<18 years | 14 additional cases |
18 to 24 years | 5 additional cases |
Decreases Compared to Placebo | |
25 to 64 years | 1 fewer case |
≥65 years | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient`s presenting symptoms.
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (Selective Serotonin Reuptake Inhibitors [SSRIs] and others) show that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in
Table 1: Risk Differences in the Number of Suicidality Cases by Age Group in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients | |
Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
Increases Compared to Placebo | |
<18 years | 14 additional cases |
18 to 24 years | 5 additional cases |
Decreases Compared to Placebo | |
25 to 64 years | 1 fewer case |
≥65 years | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient`s presenting symptoms.
Bupropion hydrochloride extended-release tablets, USP (XL) 150 mg are white to off-white, round, film coated tablets printed with “ST 150” on one side.
Bupropion hydrochloride extended-release tablets, USP (XL) 300 mg are white to off-white, round, film coated tablets printed with “ST 300” on one side.
- Bupropion hydrochloride is contraindicated in patients with seizure disorder.
- Bupropion hydrochloride is contraindicated in patients with a current or prior diagnosis of bulimia or anorexia nervosa as a higher incidence of seizures was observed in such patients treated with bupropion hydrochloride [see.]
5.3 SeizureBupropion hydrochloride extended-release tablets (XL) can cause seizure. The risk of seizure is dose-related. The dose should not exceed 300 mg once daily. Increase the dose gradually. Discontinue bupropion hydrochloride extended-release tablets (XL) and do not restart treatment if the patient experiences a seizure.
The risk of seizures is also related to patient factors, clinical situations, and concomitant medications that lower the seizure threshold. Consider these risks before initiating treatment with bupropion hydrochloride. Bupropion hydrochloride is contraindicated in patients with a seizure disorder or conditions that increase the risk of seizure (e.g., severe head injury, arteriovenous malformation, CNS tumor or CNS infection, severe stroke, anorexia nervosa or bulimia, or abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs
[see Contraindications (4)]. The following conditions can also increase the risk of seizure: concomitant use of other medications that lower the seizure threshold (e.g., other bupropion products, antipsychotics, tricyclic antidepressants, theophylline, and systemic corticosteroids), metabolic disorders (e.g., hypoglycemia, hyponatremia, severe hepatic impairment, and hypoxia), or use of illicit drugs (e.g., cocaine) or abuse or misuse of prescription drugs such as CNS stimulants. Additional predisposing conditions include diabetes mellitus treated with oral hypoglycemic drugs or insulin, use of anorectic drugs, excessive use of alcohol, benzodiazepines, sedative/hypnotics, or opiates.Incidence of Seizure with Bupropion Hydrochloride UseThe incidence of seizure with bupropion hydrochloride extended-release tablets (XL) has not been formally evaluated in clinical trials. In studies using bupropion HCl sustained-release up to 300 mg per day the incidence of seizure was approximately 0.1% (1/1,000 patients). In a large prospective, follow-up study, the seizure incidence was approximately 0.4% (13/3,200) with bupropion HCl immediate-release in the range of 300 mg to 450 mg per day.
Additional data accumulated for bupropion immediate-release suggests that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day. The risk of seizure can be reduced if the bupropion hydrochloride extended-release tablets (XL) dose does not exceed 450 mg once daily and the titration rate is gradual.
- Bupropion hydrochloride is contraindicated in patients undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs [see.,
5.3 SeizureBupropion hydrochloride extended-release tablets (XL) can cause seizure. The risk of seizure is dose-related. The dose should not exceed 300 mg once daily. Increase the dose gradually. Discontinue bupropion hydrochloride extended-release tablets (XL) and do not restart treatment if the patient experiences a seizure.
The risk of seizures is also related to patient factors, clinical situations, and concomitant medications that lower the seizure threshold. Consider these risks before initiating treatment with bupropion hydrochloride. Bupropion hydrochloride is contraindicated in patients with a seizure disorder or conditions that increase the risk of seizure (e.g., severe head injury, arteriovenous malformation, CNS tumor or CNS infection, severe stroke, anorexia nervosa or bulimia, or abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs
[see Contraindications (4)]. The following conditions can also increase the risk of seizure: concomitant use of other medications that lower the seizure threshold (e.g., other bupropion products, antipsychotics, tricyclic antidepressants, theophylline, and systemic corticosteroids), metabolic disorders (e.g., hypoglycemia, hyponatremia, severe hepatic impairment, and hypoxia), or use of illicit drugs (e.g., cocaine) or abuse or misuse of prescription drugs such as CNS stimulants. Additional predisposing conditions include diabetes mellitus treated with oral hypoglycemic drugs or insulin, use of anorectic drugs, excessive use of alcohol, benzodiazepines, sedative/hypnotics, or opiates.Incidence of Seizure with Bupropion Hydrochloride UseThe incidence of seizure with bupropion hydrochloride extended-release tablets (XL) has not been formally evaluated in clinical trials. In studies using bupropion HCl sustained-release up to 300 mg per day the incidence of seizure was approximately 0.1% (1/1,000 patients). In a large prospective, follow-up study, the seizure incidence was approximately 0.4% (13/3,200) with bupropion HCl immediate-release in the range of 300 mg to 450 mg per day.
Additional data accumulated for bupropion immediate-release suggests that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day. The risk of seizure can be reduced if the bupropion hydrochloride extended-release tablets (XL) dose does not exceed 450 mg once daily and the titration rate is gradual.
]5.3 SeizureBupropion hydrochloride extended-release tablets (XL) can cause seizure. The risk of seizure is dose-related. The dose should not exceed 300 mg once daily. Increase the dose gradually. Discontinue bupropion hydrochloride extended-release tablets (XL) and do not restart treatment if the patient experiences a seizure.
The risk of seizures is also related to patient factors, clinical situations, and concomitant medications that lower the seizure threshold. Consider these risks before initiating treatment with bupropion hydrochloride. Bupropion hydrochloride is contraindicated in patients with a seizure disorder or conditions that increase the risk of seizure (e.g., severe head injury, arteriovenous malformation, CNS tumor or CNS infection, severe stroke, anorexia nervosa or bulimia, or abrupt discontinuation of alcohol, benzodiazepines, barbiturates, and antiepileptic drugs
[see Contraindications (4)]. The following conditions can also increase the risk of seizure: concomitant use of other medications that lower the seizure threshold (e.g., other bupropion products, antipsychotics, tricyclic antidepressants, theophylline, and systemic corticosteroids), metabolic disorders (e.g., hypoglycemia, hyponatremia, severe hepatic impairment, and hypoxia), or use of illicit drugs (e.g., cocaine) or abuse or misuse of prescription drugs such as CNS stimulants. Additional predisposing conditions include diabetes mellitus treated with oral hypoglycemic drugs or insulin, use of anorectic drugs, excessive use of alcohol, benzodiazepines, sedative/hypnotics, or opiates.Incidence of Seizure with Bupropion Hydrochloride UseThe incidence of seizure with bupropion hydrochloride extended-release tablets (XL) has not been formally evaluated in clinical trials. In studies using bupropion HCl sustained-release up to 300 mg per day the incidence of seizure was approximately 0.1% (1/1,000 patients). In a large prospective, follow-up study, the seizure incidence was approximately 0.4% (13/3,200) with bupropion HCl immediate-release in the range of 300 mg to 450 mg per day.
Additional data accumulated for bupropion immediate-release suggests that the estimated seizure incidence increases almost tenfold between 450 and 600 mg/day. The risk of seizure can be reduced if the bupropion hydrochloride extended-release tablets (XL) dose does not exceed 450 mg once daily and the titration rate is gradual.
- The use of MAOIs (intended to treat psychiatric disorders) concomitantly with bupropion hydrochloride or within 14 days of discontinuing treatment with bupropion hydrochloride is contraindicated. There is an increased risk of hypertensive reactions when bupropion hydrochloride is used concomitantly with MAOIs. The use of bupropion hydrochloride within 14 days of discontinuing treatment with an MAOI is also contraindicated. Starting bupropion hydrochloride in a patient treated with reversible MAOIs such as linezolid or intravenous methylene blue is contraindicated. [see.,
2.9 Use of Bupropion Hydrochloride Extended-release Tablets (XL) with Reversible MAOIs such as Linezolid or Methylene BlueDo not start bupropion hydrochloride extended-release tablets (XL) in a patient who is being treated with a reversible MAOI such as linezolid or intravenous methylene blue. Drug interactions can increase risk of hypertensive reactions. In a patient who requires more urgent treatment of a psychiatric condition, non-pharmacological interventions, including hospitalization, should be considered
[see Contraindications(4)].
In some cases, a patient already receiving therapy with bupropion hydrochloride extended-release tablets (XL) may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of hypertensive reactions in a particular patient, bupropion hydrochloride extended-release tablets (XL) should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for 2 weeks or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with bupropion hydrochloride extended-release tablets (XL) may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue.
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg per kg with bupropion hydrochloride extended-release tablets (XL) is unclear. The clinician should, nevertheless, be aware of the possibility of a drug interaction with such use[see Contraindications(4), Drug Interactions (7.6)].,5.4 HypertensionTreatment with bupropion hydrochloride can result in elevated blood pressure and hypertension.
Assess blood pressure before initiating treatment with bupropion hydrochloride, and monitor periodically during treatment. The risk of hypertension is increased if bupropion hydrochloride is used concomitantly with MAOIs or other drugs that increase dopaminergic or noradrenergic activity
[see Contraindications (4)].Data from a comparative trial of the sustained-release formulation of bupropion HCl, nicotine transdermal system (NTS), the combination of sustained-release bupropion plus NTS, and placebo as an aid to smoking cessation suggest a higher incidence of treatment-emergent hypertension in patients treated with the combination of sustained-release bupropion and NTS. In this trial, 6.1% of subjects treated with the combination of sustained-release bupropion and NTS had treatment-emergent hypertension compared to 2.5%, 1.6%, and 3.1% of subjects treated with sustained-release bupropion, NTS, and placebo, respectively. The majority of these subjects had evidence of pre-existing hypertension. Three subjects (1.2%) treated with the combination of sustained-release bupropion and NTS and 1 subject (0.4%) treated with NTS had study medication discontinued due to hypertension compared with none of the subjects treated with sustained-release bupropion or placebo. Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement.
In the 3 trials of bupropion HCl extended-release in seasonal affective disorder, there were significant elevations in blood pressure. Hypertension was reported as an adverse reaction for 2% of the bupropion group (11/537) and none in the placebo group (0/511). In the SAD trials, 2 patients treated with bupropion discontinued from the study because they developed hypertension. None of the placebo group discontinued because of hypertension. The mean increase in systolic blood pressure was 1.3 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was statistically significant (p=0.013). The mean increase in diastolic blood pressure was 0.8 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was not statistically significant (p=0.075). In the SAD trials, 82% of patients were treated with 300 mg per day, and 18% were treated with 150 mg per day. The mean daily dose was 270 mg per day. The mean duration of bupropion exposure was 126 days.
In a clinical trial of bupropion immediate-release in MDD subjects with stable congestive heart failure (CHF) (N=36), bupropion was associated with an exacerbation of pre-existing hypertension in 2 subjects, leading to discontinuation of bupropion treatment. There are no controlled studies assessing the safety of bupropion in patients with a recent history of myocardial infarction or unstable cardiac disease.
]7.6 MAO InhibitorsBupropion inhibits the reuptake of dopamine and norepinephrine. Concomitant use of MAOIs and bupropion is contraindicated because there is an increased risk of hypertensive reactions if bupropion is used concomitantly with MAOIs. Studies in animals demonstrate that the acute toxicity of bupropion is enhanced by the MAO inhibitor phenelzine. At least 14 days should elapse between discontinuation of an MAOI intended to treat depression and initiation of treatment with bupropion hydrochloride. Conversely, at least 14 days should be allowed after stopping bupropion hydrochloride before starting an MAOI antidepressant [
see Dosage and Administration , Contraindications (4)]. - Bupropion hydrochloride extended-release tablets (XL) is contraindicated in patients with known hypersensitivity to bupropion or other ingredients of bupropion hydrochloride extended-release tablets (XL). Anaphylactoid/anaphylactic reactions and Stevens-Johnson syndrome have been reported [see.]
5.8 Hypersensitivity ReactionsAnaphylactoid/anaphylactic reactions have occurred during clinical trials with bupropion. Reactions have been characterized by pruritus, urticaria, angioedema, and dyspnea, requiring medical treatment. In addition, there have been rare, spontaneous postmarketing reports of erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock associated with bupropion. Instruct patients to discontinue bupropion hydrochloride and consult a healthcare provider if they develop an allergic or anaphylactoid/anaphylactic reaction (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
There are reports of arthralgia, myalgia, fever with rash and other symptoms of serum sickness suggestive of delayed hypersensitivity.
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Suicidal thoughts and behaviors in children, adolescents, and young adults [see]
5.1 Suicidal Thoughts and Behaviors in Children, Adolescents, and Young AdultsPatients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment.
Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (Selective Serotonin Reuptake Inhibitors [SSRIs] and others) show that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included a total of 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in
Table 1.Table 1: Risk Differences in the Number of Suicidality Cases by Age Group in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult PatientsAge RangeDrug-Placebo Difference in Number of Cases ofSuicidality per 1,000 Patients TreatedIncreases Compared to Placebo
<18 years
14 additional cases
18 to 24 years
5 additional cases
Decreases Compared to Placebo
25 to 64 years
1 fewer case
≥65 years
6 fewer cases
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases[see Boxed Warning, Use in Specific Populations (8.4)].The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient`s presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for bupropion hydrochloride should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose. - Neuropsychiatric adverse events and suicide risk in smoking cessation treatment [see]
5.2 Neuropsychiatric Adverse Events and Suicide Risk in Smoking Cessation TreatmentBupropion hydrochloride extended-release tablets (XL) is not approved for smoking cessation treatment; however, bupropion HCl sustained-release is approved for this use. Serious neuropsychiatric adverse events have been reported in patients taking bupropion for smoking cessation. These postmarketing reports have included changes in mood (including depression and mania), psychosis, hallucinations, paranoia, delusions, homicidal ideation, aggression, hostility, agitation, anxiety, and panic, as well as suicidal ideation, suicide attempt, and completed suicide
[see Adverse Reactions (6.2)].Some patients who stopped smoking may have been experiencing symptoms of nicotine withdrawal, including depressed mood. Depression, rarely including suicidal ideation, has been reported in smokers undergoing a smoking cessation attempt without medication. However, some of these adverse events occurred in patients taking bupropion who continued to smoke.Neuropsychiatric adverse events occurred in patients without and with pre-existing psychiatric disease; some patients experienced worsening of their psychiatric illnesses. Observe patients for the occurrence of neuropsychiatric adverse events. Advise patients and caregivers that the patient should stop taking bupropion hydrochloride and contact a healthcare provider immediately if agitation, depressed mood, or changes in behavior or thinking that are not typical for the patient are observed, or if the patient develops suicidal ideation or suicidal behavior. The healthcare provider should evaluate the severity of the adverse events and the extent to which the patient is benefiting from treatment, and consider options including continued treatment under closer monitoring, or discontinuing treatment. In many postmarketing cases, resolution of symptoms after discontinuation of bupropion was reported. However, the symptoms persisted in some cases; therefore, ongoing monitoring and supportive care should be provided until symptoms resolve.
- Seizure [see]
5.4 HypertensionTreatment with bupropion hydrochloride can result in elevated blood pressure and hypertension.
Assess blood pressure before initiating treatment with bupropion hydrochloride, and monitor periodically during treatment. The risk of hypertension is increased if bupropion hydrochloride is used concomitantly with MAOIs or other drugs that increase dopaminergic or noradrenergic activity
[see Contraindications (4)].Data from a comparative trial of the sustained-release formulation of bupropion HCl, nicotine transdermal system (NTS), the combination of sustained-release bupropion plus NTS, and placebo as an aid to smoking cessation suggest a higher incidence of treatment-emergent hypertension in patients treated with the combination of sustained-release bupropion and NTS. In this trial, 6.1% of subjects treated with the combination of sustained-release bupropion and NTS had treatment-emergent hypertension compared to 2.5%, 1.6%, and 3.1% of subjects treated with sustained-release bupropion, NTS, and placebo, respectively. The majority of these subjects had evidence of pre-existing hypertension. Three subjects (1.2%) treated with the combination of sustained-release bupropion and NTS and 1 subject (0.4%) treated with NTS had study medication discontinued due to hypertension compared with none of the subjects treated with sustained-release bupropion or placebo. Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement.
In the 3 trials of bupropion HCl extended-release in seasonal affective disorder, there were significant elevations in blood pressure. Hypertension was reported as an adverse reaction for 2% of the bupropion group (11/537) and none in the placebo group (0/511). In the SAD trials, 2 patients treated with bupropion discontinued from the study because they developed hypertension. None of the placebo group discontinued because of hypertension. The mean increase in systolic blood pressure was 1.3 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was statistically significant (p=0.013). The mean increase in diastolic blood pressure was 0.8 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was not statistically significant (p=0.075). In the SAD trials, 82% of patients were treated with 300 mg per day, and 18% were treated with 150 mg per day. The mean daily dose was 270 mg per day. The mean duration of bupropion exposure was 126 days.
In a clinical trial of bupropion immediate-release in MDD subjects with stable congestive heart failure (CHF) (N=36), bupropion was associated with an exacerbation of pre-existing hypertension in 2 subjects, leading to discontinuation of bupropion treatment. There are no controlled studies assessing the safety of bupropion in patients with a recent history of myocardial infarction or unstable cardiac disease.
- Hypertension [see]
5.4 HypertensionTreatment with bupropion hydrochloride can result in elevated blood pressure and hypertension.
Assess blood pressure before initiating treatment with bupropion hydrochloride, and monitor periodically during treatment. The risk of hypertension is increased if bupropion hydrochloride is used concomitantly with MAOIs or other drugs that increase dopaminergic or noradrenergic activity
[see Contraindications (4)].Data from a comparative trial of the sustained-release formulation of bupropion HCl, nicotine transdermal system (NTS), the combination of sustained-release bupropion plus NTS, and placebo as an aid to smoking cessation suggest a higher incidence of treatment-emergent hypertension in patients treated with the combination of sustained-release bupropion and NTS. In this trial, 6.1% of subjects treated with the combination of sustained-release bupropion and NTS had treatment-emergent hypertension compared to 2.5%, 1.6%, and 3.1% of subjects treated with sustained-release bupropion, NTS, and placebo, respectively. The majority of these subjects had evidence of pre-existing hypertension. Three subjects (1.2%) treated with the combination of sustained-release bupropion and NTS and 1 subject (0.4%) treated with NTS had study medication discontinued due to hypertension compared with none of the subjects treated with sustained-release bupropion or placebo. Monitoring of blood pressure is recommended in patients who receive the combination of bupropion and nicotine replacement.
In the 3 trials of bupropion HCl extended-release in seasonal affective disorder, there were significant elevations in blood pressure. Hypertension was reported as an adverse reaction for 2% of the bupropion group (11/537) and none in the placebo group (0/511). In the SAD trials, 2 patients treated with bupropion discontinued from the study because they developed hypertension. None of the placebo group discontinued because of hypertension. The mean increase in systolic blood pressure was 1.3 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was statistically significant (p=0.013). The mean increase in diastolic blood pressure was 0.8 mmHg in the bupropion group and 0.1 mmHg in the placebo group. The difference was not statistically significant (p=0.075). In the SAD trials, 82% of patients were treated with 300 mg per day, and 18% were treated with 150 mg per day. The mean daily dose was 270 mg per day. The mean duration of bupropion exposure was 126 days.
In a clinical trial of bupropion immediate-release in MDD subjects with stable congestive heart failure (CHF) (N=36), bupropion was associated with an exacerbation of pre-existing hypertension in 2 subjects, leading to discontinuation of bupropion treatment. There are no controlled studies assessing the safety of bupropion in patients with a recent history of myocardial infarction or unstable cardiac disease.
- Activation of mania or hypomania [see]
5.5 Activation of Mania/HypomaniaAntidepressant treatment can precipitate a manic, mixed, or hypomanic manic episode. The risk appears to be increased in patients with bipolar disorder or who have risk factors for bipolar disorder. Prior to initiating bupropion hydrochloride, screen patients for a history of bipolar disorder and the presence of risk factors for bipolar disorder (e.g., family history of bipolar disorder, suicide, or depression). Bupropion hydrochloride is not approved for the treatment of bipolar depression.
- Psychosis and other neuropsychiatric events [see]
5.6 Psychosis and Other Neuropsychiatric ReactionsDepressed patients treated with bupropion have had a variety of neuropsychiatric signs and symptoms, including delusions, hallucinations, psychosis, concentration disturbance, paranoia, and confusion. Some of these patients had a diagnosis of bipolar disorder. In some cases, these symptoms abated upon dose reduction and/or withdrawal of treatment. Discontinue bupropion hydrochloride if these reactions occur.
- Angle-Closure Glaucoma [see]
5.7 Angle-Closure GlaucomaAngle-Closure Glaucoma: The pupillary dilation that occurs following use of many antidepressant drugs including bupropion hydrochloride may trigger an angle-closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
- Hypersensitivity reactions [see]
5.8 Hypersensitivity ReactionsAnaphylactoid/anaphylactic reactions have occurred during clinical trials with bupropion. Reactions have been characterized by pruritus, urticaria, angioedema, and dyspnea, requiring medical treatment. In addition, there have been rare, spontaneous postmarketing reports of erythema multiforme, Stevens-Johnson syndrome, and anaphylactic shock associated with bupropion. Instruct patients to discontinue bupropion hydrochloride and consult a healthcare provider if they develop an allergic or anaphylactoid/anaphylactic reaction (e.g., skin rash, pruritus, hives, chest pain, edema, and shortness of breath) during treatment.
There are reports of arthralgia, myalgia, fever with rash and other symptoms of serum sickness suggestive of delayed hypersensitivity.
Bupropion hydrochloride extended-release tablets (XL), an antidepressant of the aminoketone class, is chemically unrelated to tricyclic, tetracyclic, selective serotonin reuptake inhibitor, or other known antidepressant agents. Its structure closely resembles that of diethylpropion; it is related to phenylethylamines. It is designated as (±)-1-(3-chorophenyl)-2-[(1,1-dimethylethyl)amino]-1-propanone hydrochloride. The molecular weight is 276.2. The molecular formula is C
13H
18ClNO·HCl. Bupropion hydrochloride powder is white, crystalline, and highly soluble in water. It has a bitter taste and produces the sensation of local anesthesia on the oral mucosa. The structural formula is:
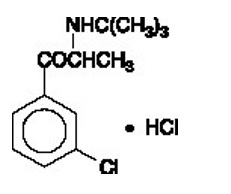
Bupropion hydrochloride extended-release tablets, USP (XL) are supplied for oral administration as 150 mg and 300 mg white to off-white extended-release tablets. Each tablet contains the labeled amount of bupropion hydrochloride, USP and the inactive ingredients: povidone, tartaric acid, glyceryl distearate, magnesium stearate, hydroxypropyl cellulose, ethylcellulose, methacrylic acid copolymer dispersion and colloidal silicon dioxide. The tablets are printed with black ink comprising of shellac glaze (modified) in SD-45, isopropyl alcohol, black iron oxide non-irradiated, n-butyl alcohol, propylene glycol and ammonium hydroxide.
The insoluble shell of the extended-release tablet may remain intact during gastrointestinal transit and is eliminated in the feces.
Meets USP Dissolution Test #4.
Bupropion hydrochloride extended-release tablets, USP (XL) are spplied as:
NDC | Strength | Quantity | Description |
62135-875-30 | 150 mg | bottle of 30 tablets | white to off-white, round, film coated tablets printed with “ST 150” on one side |
62135-876-30 | 300 mg | bottle of 30 tablets | white to off-white, round, film coated tablets printed with “ST 300” on one side |
Bupropion hydrochloride extended-release tablets (XL) may have an odor.