Cabergoline
Cabergoline Prescribing Information
Cabergoline tablets are indicated for the treatment of hyperprolactinemic disorders, either idiopathic or due to pituitary adenomas.
The recommended dosage of cabergoline tablets for initiation of therapy is 0.25 mg twice a week. Dosage may be increased by 0.25 mg twice weekly up to a dosage of 1 mg twice a week according to the patient’s serum prolactin level. Before initiating treatment, cardiovascular evaluation should be performed and echocardiography should be considered to assess for valvular disease.
Dosage increases should not occur more rapidly than every 4 weeks, so that the physician can assess the patient’s response to each dosage level. If the patient does not respond adequately, and no additional benefit is observed with higher doses, the lowest dose that achieved maximal response should be used and other therapeutic approaches considered. Patients receiving long‑term treatment with cabergoline should undergo periodic assessment of their cardiac status and echocardiography should be considered.
After a normal serum prolactin level has been maintained for 6 months, cabergoline may be discontinued, with periodic monitoring of the serum prolactin level to determine whether or when treatment with cabergoline should be reinstituted. The durability of efficacy beyond 24 months of therapy with cabergoline has not been established.
Cabergoline tablets are contraindicated in patients with:
- Uncontrolled hypertension or known hypersensitivity to ergot derivatives.
- History of cardiac valvular disorders, as suggested by anatomical evidence of valvulopathy of any valve, determined by pre-treatment evaluation including echocardiographic demonstration of valve leaflet thickening, valve restriction, or mixed valve restriction‑stenosis. (See )
WARNINGS1. PregnancyDopamine agonists in general should not be used in patients with pregnancy-induced hypertension, for example, preeclampsia, eclampsia, and postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.
2.000000000000000e+00
Fibrotic Complicationsa. Cardiac ValvulopathyAll patients should undergo a cardiovascular evaluation, including echocardiogram to assess the potential presence of valvular disease. If valvular disease is detected, the patient should not be treated with cabergoline. (See
CONTRAINDICATIONS) Postmarketing cases of cardiac valvulopathy have been reported in patients receiving cabergoline. These cases have generally occurred during administration of high doses of cabergoline (> 2mg/day) for the treatment of Parkinson’s disease. Cases of cardiac valvulopathy have also been reported in patients receiving lower doses of cabergoline for the treatment of hyperprolactinemic disorders.A multi-country, retrospective cohort study using general practice records and record linkage systems in the UK, Italy and the Netherlands was conducted to assess the association between new use of dopamine agonists including cabergoline (n = 27,812) for Parkinson’s disease and hyperprolactinemia and cardiac valvular regurgitation (CVR), other fibroses, and other cardiopulmonary events over a maximum of 12 years of follow up. In this study, the use of cabergoline among persons with Parkinson's disease was associated with an increased risk of CVR when compared to non-ergot-derived dopamine agonists (DAs) and levodopa [Incidence Rate (IR) per 10,000 person years of 68.1 (95% confidence interval (CI): 37.2 to 115.3) for cabergoline vs. 10 (95% CI: 5.2 to 19.4) for non-ergot DAs and 11.3 (95% CI: 7.2 to 17) for levodopa]. In the study analysis confined to persons with dopamine agonist-treated hyperprolactinemia (n = 8,386), when compared to non-use (n = 15,147), persons exposed to cabergoline did not have an elevated risk of CVR. The findings with respect to the risk of CVR associated with cabergoline treatment for persons with Parkinson’s disease (increased risk) and those with hyperprolactinemia (no increased risk) are consistent with the findings in other published studies.
Physicians should use the lowest effective dose of cabergoline for the treatment of hyperprolactinemic disorders and should periodically reassess the need for continuing therapy with cabergoline. Following treatment initiation, clinical and diagnostic monitoring (for example, chest x-ray, CT scan and cardiac echocardiogram) should be conducted to assess the risk of cardiac valvulopathy. The recommended frequency of routine echocardiographic monitoring is every 6 months to 12 months or as clinically indicated with the presence of signs and symptoms such as edema, new cardiac murmur, dyspnea, or congestive heart failure.
Cabergoline should be discontinued if an echocardiogram reveals new valvular regurgitation, valvular restriction or valve leaflet thickening.
Cabergoline should be used with caution in patients exposed to other medications associated with valvulopathy.
b. Extracardiac Fibrotic ReactionsPostmarketing cases of pleural, pericardial, and retroperitoneal fibrosis have been reported following administration of cabergoline. Some reports were in patients previously treated with other ergotinic dopamine agonists. Cabergoline should not be used in patients with a history of cardiac or extracardiac fibrotic disorders.
Fibrotic disorders can have an insidious onset and patients should be monitored for manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of:
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.
Clinical and diagnostic monitoring such as erythrocyte sedimentation rate, chest-x ray, serum creatinine measurements, and other investigations should be considered at baseline and as necessary while patients are treated with cabergoline.
Following diagnosis of pleural effusion or pulmonary fibrosis, the discontinuance of cabergoline was reported to result in improvement of signs and symptoms.
- History of pulmonary, pericardial, or retroperitoneal fibrotic disorders. (See )
WARNINGS1. PregnancyDopamine agonists in general should not be used in patients with pregnancy-induced hypertension, for example, preeclampsia, eclampsia, and postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.
2.000000000000000e+00
Fibrotic Complicationsa. Cardiac ValvulopathyAll patients should undergo a cardiovascular evaluation, including echocardiogram to assess the potential presence of valvular disease. If valvular disease is detected, the patient should not be treated with cabergoline. (See
CONTRAINDICATIONS) Postmarketing cases of cardiac valvulopathy have been reported in patients receiving cabergoline. These cases have generally occurred during administration of high doses of cabergoline (> 2mg/day) for the treatment of Parkinson’s disease. Cases of cardiac valvulopathy have also been reported in patients receiving lower doses of cabergoline for the treatment of hyperprolactinemic disorders.A multi-country, retrospective cohort study using general practice records and record linkage systems in the UK, Italy and the Netherlands was conducted to assess the association between new use of dopamine agonists including cabergoline (n = 27,812) for Parkinson’s disease and hyperprolactinemia and cardiac valvular regurgitation (CVR), other fibroses, and other cardiopulmonary events over a maximum of 12 years of follow up. In this study, the use of cabergoline among persons with Parkinson's disease was associated with an increased risk of CVR when compared to non-ergot-derived dopamine agonists (DAs) and levodopa [Incidence Rate (IR) per 10,000 person years of 68.1 (95% confidence interval (CI): 37.2 to 115.3) for cabergoline vs. 10 (95% CI: 5.2 to 19.4) for non-ergot DAs and 11.3 (95% CI: 7.2 to 17) for levodopa]. In the study analysis confined to persons with dopamine agonist-treated hyperprolactinemia (n = 8,386), when compared to non-use (n = 15,147), persons exposed to cabergoline did not have an elevated risk of CVR. The findings with respect to the risk of CVR associated with cabergoline treatment for persons with Parkinson’s disease (increased risk) and those with hyperprolactinemia (no increased risk) are consistent with the findings in other published studies.
Physicians should use the lowest effective dose of cabergoline for the treatment of hyperprolactinemic disorders and should periodically reassess the need for continuing therapy with cabergoline. Following treatment initiation, clinical and diagnostic monitoring (for example, chest x-ray, CT scan and cardiac echocardiogram) should be conducted to assess the risk of cardiac valvulopathy. The recommended frequency of routine echocardiographic monitoring is every 6 months to 12 months or as clinically indicated with the presence of signs and symptoms such as edema, new cardiac murmur, dyspnea, or congestive heart failure.
Cabergoline should be discontinued if an echocardiogram reveals new valvular regurgitation, valvular restriction or valve leaflet thickening.
Cabergoline should be used with caution in patients exposed to other medications associated with valvulopathy.
b. Extracardiac Fibrotic ReactionsPostmarketing cases of pleural, pericardial, and retroperitoneal fibrosis have been reported following administration of cabergoline. Some reports were in patients previously treated with other ergotinic dopamine agonists. Cabergoline should not be used in patients with a history of cardiac or extracardiac fibrotic disorders.
Fibrotic disorders can have an insidious onset and patients should be monitored for manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of:
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.
Clinical and diagnostic monitoring such as erythrocyte sedimentation rate, chest-x ray, serum creatinine measurements, and other investigations should be considered at baseline and as necessary while patients are treated with cabergoline.
Following diagnosis of pleural effusion or pulmonary fibrosis, the discontinuance of cabergoline was reported to result in improvement of signs and symptoms.
The safety of cabergoline tablets has been evaluated in more than 900 patients with hyperprolactinemic disorders. Most adverse events were mild or moderate in severity.
In a 4-week, double-blind, placebo-controlled study, treatment consisted of placebo or cabergoline at fixed doses of 0.125 mg, 0.5 mg, 0.75 mg, or 1 mg twice weekly. Doses were halved during the first week. Since a possible dose-related effect was observed for nausea only, the four cabergoline treatment groups have been combined. The incidence of the most common adverse events during the placebo-controlled study is presented in the following table.
Adverse Event* | Cabergoline (n = 168) 0.125 to 1 mg two times a week | Placebo (n = 20) |
Number (percent) | ||
Gastrointestinal Nausea Constipation Abdominal pain Dyspepsia Vomiting | 45 (27) 16 (10) 9 (5) 4 (2) 4 (2) | 4 (20) 0 1 (5) 0 0 |
Central and Peripheral Nervous System Headache Dizziness Paresthesia Vertigo | 43 (26) 25 (15) 2 (1) 2 (1) | 5 (25) 1 (5) 0 0 |
Body As a Whole Asthenia Fatigue Hot flashes | 15 (9) 12 (7) 2 (1) | 2 (10) 0 1 (5) |
Psychiatric Somnolence Depression Nervousness | 9 (5) 5 (3) 4 (2) | 1 (5) 1 (5) 0 |
Autonomic Nervous System Postural hypotension | 6 (4) | 0 |
Reproductive – Female Breast pain Dysmenorrhea | 2 (1) 2 (1) | 0 0 |
Vision Abnormal vision | 2 (1) | 0 |
*Reported at ≥ 1% for cabergoline
In the 8-week, double-blind period of the comparative trial with bromocriptine, cabergoline (at a dose of 0.5 mg twice weekly) was discontinued because of an adverse event in 4 of 221 patients (2%) while bromocriptine (at a dose of 2.5 mg two times a day) was discontinued in 14 of 231 patients (6%). The most common reasons for discontinuation from cabergoline were headache, nausea and vomiting (3 patients, 2 patients and 2 patients respectively); the most common reasons for discontinuation from bromocriptine were nausea, vomiting, headache, and dizziness or vertigo (10 patients, 3 patients, 3 patients, and 3 patients respectively). The incidence of the most common adverse events during the double-blind portion of the comparative trial with bromocriptine is presented in the following table.
| Adverse Event* | Cabergoline(n = 221) | Bromocriptine (n = 231) | |
|---|---|---|---|
| Number (percent) | |||
Gastrointestinal | |||
Nausea | 63 (29) | 100 (43) | |
Constipation | 15 (7) | 21 (9) | |
Abdominal pain | 12 (5) | 19 (8) | |
Dyspepsia | 11 (5) | 16 (7) | |
Vomiting | 9 (4) | 16 (7) | |
Dry mouth | 5 (2) | 2 (1) | |
Diarrhea | 4 (2) | 7 (3) | |
Flatulence | 4 (2) | 3 (1) | |
Throat irritation | 2 (1) | 0 | |
Toothache | 2 (1) | 0 | |
Central and Peripheral Nervous System | |||
Headache | 58 (26) | 62 (27) | |
Dizziness | 38 (17) | 42 (18) | |
Vertigo | 9 (4) | 10 (4) | |
Paresthesia | 5 (2) | 6 (3) | |
Body As a Whole | |||
Asthenia | 13 (6) | 15 (6) | |
Fatigue | 10 (5) | 18 (8) | |
Syncope | 3 (1) | 3 (1) | |
Influenza-like symptoms | 2 (1) | 0 | |
Malaise | 2 (1) | 0 | |
Periorbital edema | 2 (1) | 2 (1) | |
Peripheral edema | 2 (1) | 1 | |
Psychiatric | |||
Depression | 7 (3) | 5 (2) | |
Somnolence | 5 (2) | 5 (2) | |
Anorexia | 3 (1) | 3 (1) | |
Anxiety | 3 (1) | 3 (1) | |
Insomnia | 3 (1) | 2 (1) | |
Impaired concentration | 2 (1) | 1 | |
Nervousness | 2 (1) | 5 (2) | |
Cardiovascular Hot flashes Hypotension Dependent edema Palpitation | 6 (3) 3 (1) 2 (1) 2 (1) | 3 (1) 4 (2) 1 5 (2) | |
Reproductive – Female Breast pain Dysmenorrhea | 5 (2) 2 (1) | 8 (3) 1 | |
Skin and Appendages Acne Pruritus | 3 (1) 2 (1) | 0 1 | |
Musculoskeletal Pain Arthralgia | 4 (2) 2 (1) | 6 (3) 0 | |
Respiratory Rhinitis | 2 (1) | 9 (4) | |
Vision Abnormal vision | 2 (1) | 2 (1) | |
*Reported at ≥ 1% for cabergoline
Other adverse events that were reported at an incidence of < 1% in the overall clinical studies follow.
The safety of cabergoline has been evaluated in approximately 1,200 patients with Parkinson’s disease in controlled and uncontrolled studies at dosages of up to 11.5 mg/day which greatly exceeds the maximum recommended dosage of cabergoline for hyperprolactinemic disorders. In addition to the adverse events that occurred in the patients with hyperprolactinemic disorders, the most common adverse events in patients with Parkinson’s disease were dyskinesia, hallucinations, confusion, and peripheral edema. Heart failure, pleural effusion, pulmonary fibrosis, and gastric or duodenal ulcer occurred rarely. One case of constrictive pericarditis has been reported.
The following events have been reported in association with cabergoline: cardiac valvulopathy and extracardiac fibrotic reactions, (See
Dopamine agonists in general should not be used in patients with pregnancy-induced hypertension, for example, preeclampsia, eclampsia, and postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.
2.000000000000000e+00
All patients should undergo a cardiovascular evaluation, including echocardiogram to assess the potential presence of valvular disease. If valvular disease is detected, the patient should not be treated with cabergoline. (See
A multi-country, retrospective cohort study using general practice records and record linkage systems in the UK, Italy and the Netherlands was conducted to assess the association between new use of dopamine agonists including cabergoline (n = 27,812) for Parkinson’s disease and hyperprolactinemia and cardiac valvular regurgitation (CVR), other fibroses, and other cardiopulmonary events over a maximum of 12 years of follow up. In this study, the use of cabergoline among persons with Parkinson's disease was associated with an increased risk of CVR when compared to non-ergot-derived dopamine agonists (DAs) and levodopa [Incidence Rate (IR) per 10,000 person years of 68.1 (95% confidence interval (CI): 37.2 to 115.3) for cabergoline vs. 10 (95% CI: 5.2 to 19.4) for non-ergot DAs and 11.3 (95% CI: 7.2 to 17) for levodopa]. In the study analysis confined to persons with dopamine agonist-treated hyperprolactinemia (n = 8,386), when compared to non-use (n = 15,147), persons exposed to cabergoline did not have an elevated risk of CVR. The findings with respect to the risk of CVR associated with cabergoline treatment for persons with Parkinson’s disease (increased risk) and those with hyperprolactinemia (no increased risk) are consistent with the findings in other published studies.
Physicians should use the lowest effective dose of cabergoline for the treatment of hyperprolactinemic disorders and should periodically reassess the need for continuing therapy with cabergoline. Following treatment initiation, clinical and diagnostic monitoring (for example, chest x-ray, CT scan and cardiac echocardiogram) should be conducted to assess the risk of cardiac valvulopathy. The recommended frequency of routine echocardiographic monitoring is every 6 months to 12 months or as clinically indicated with the presence of signs and symptoms such as edema, new cardiac murmur, dyspnea, or congestive heart failure.
Cabergoline should be discontinued if an echocardiogram reveals new valvular regurgitation, valvular restriction or valve leaflet thickening.
Cabergoline should be used with caution in patients exposed to other medications associated with valvulopathy.
Postmarketing cases of pleural, pericardial, and retroperitoneal fibrosis have been reported following administration of cabergoline. Some reports were in patients previously treated with other ergotinic dopamine agonists. Cabergoline should not be used in patients with a history of cardiac or extracardiac fibrotic disorders.
Fibrotic disorders can have an insidious onset and patients should be monitored for manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of:
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.
Clinical and diagnostic monitoring such as erythrocyte sedimentation rate, chest-x ray, serum creatinine measurements, and other investigations should be considered at baseline and as necessary while patients are treated with cabergoline.
Following diagnosis of pleural effusion or pulmonary fibrosis, the discontinuance of cabergoline was reported to result in improvement of signs and symptoms.
Dopamine agonists in general should not be used in patients with pregnancy-induced hypertension, for example, preeclampsia, eclampsia, and postpartum hypertension, unless the potential benefit is judged to outweigh the possible risk.
2.000000000000000e+00
All patients should undergo a cardiovascular evaluation, including echocardiogram to assess the potential presence of valvular disease. If valvular disease is detected, the patient should not be treated with cabergoline. (See
A multi-country, retrospective cohort study using general practice records and record linkage systems in the UK, Italy and the Netherlands was conducted to assess the association between new use of dopamine agonists including cabergoline (n = 27,812) for Parkinson’s disease and hyperprolactinemia and cardiac valvular regurgitation (CVR), other fibroses, and other cardiopulmonary events over a maximum of 12 years of follow up. In this study, the use of cabergoline among persons with Parkinson's disease was associated with an increased risk of CVR when compared to non-ergot-derived dopamine agonists (DAs) and levodopa [Incidence Rate (IR) per 10,000 person years of 68.1 (95% confidence interval (CI): 37.2 to 115.3) for cabergoline vs. 10 (95% CI: 5.2 to 19.4) for non-ergot DAs and 11.3 (95% CI: 7.2 to 17) for levodopa]. In the study analysis confined to persons with dopamine agonist-treated hyperprolactinemia (n = 8,386), when compared to non-use (n = 15,147), persons exposed to cabergoline did not have an elevated risk of CVR. The findings with respect to the risk of CVR associated with cabergoline treatment for persons with Parkinson’s disease (increased risk) and those with hyperprolactinemia (no increased risk) are consistent with the findings in other published studies.
Physicians should use the lowest effective dose of cabergoline for the treatment of hyperprolactinemic disorders and should periodically reassess the need for continuing therapy with cabergoline. Following treatment initiation, clinical and diagnostic monitoring (for example, chest x-ray, CT scan and cardiac echocardiogram) should be conducted to assess the risk of cardiac valvulopathy. The recommended frequency of routine echocardiographic monitoring is every 6 months to 12 months or as clinically indicated with the presence of signs and symptoms such as edema, new cardiac murmur, dyspnea, or congestive heart failure.
Cabergoline should be discontinued if an echocardiogram reveals new valvular regurgitation, valvular restriction or valve leaflet thickening.
Cabergoline should be used with caution in patients exposed to other medications associated with valvulopathy.
Postmarketing cases of pleural, pericardial, and retroperitoneal fibrosis have been reported following administration of cabergoline. Some reports were in patients previously treated with other ergotinic dopamine agonists. Cabergoline should not be used in patients with a history of cardiac or extracardiac fibrotic disorders.
Fibrotic disorders can have an insidious onset and patients should be monitored for manifestations of progressive fibrosis. Therefore, during treatment, attention should be paid to the signs and symptoms of:
- Pleuro-pulmonary disease such as dyspnea, shortness of breath, persistent cough or chest pain.
- Renal insufficiency or ureteral/abdominal vascular obstruction that may occur with pain in the loin/flank and lower limb edema as well as any possible abdominal masses or tenderness that may indicate retroperitoneal fibrosis.
- Cardiac failure: Cases of valvular and pericardial fibrosis have often manifested as cardiac failure. Therefore, valvular fibrosis (and constrictive pericarditis) should be excluded if such symptoms occur.
Clinical and diagnostic monitoring such as erythrocyte sedimentation rate, chest-x ray, serum creatinine measurements, and other investigations should be considered at baseline and as necessary while patients are treated with cabergoline.
Following diagnosis of pleural effusion or pulmonary fibrosis, the discontinuance of cabergoline was reported to result in improvement of signs and symptoms.
Other events have been reported in association with cabergoline: impulse control/compulsive behavior symptoms, including hypersexuality, increased libido and pathological gambling (See
Impulse control/compulsive behaviors, including pathological gambling, increased libido, and hypersexuality have been reported in patients treated with dopamine agonists including cabergoline. This has been generally reversible upon reduction of the dose or treatment discontinuation (See
Cabergoline should not be administered concurrently with D2-antagonists, such as phenothiazines, butyrophenones, thioxanthenes, or metoclopramide.
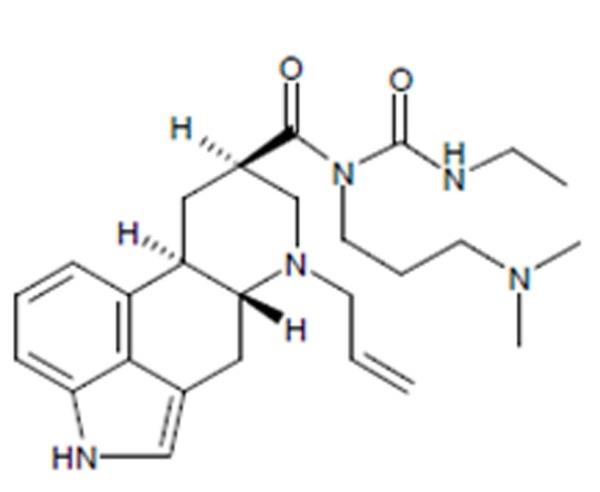
Cabergoline tablets, USP contain cabergoline, USP a dopamine receptor agonist. The chemical name for cabergoline is 1-[(6-allylergolin-8β-yl)-carbonyl]-1-[3-(dimethylamino) propyl]-3-ethylurea. Its molecular formula is C26H37N5O2, and its molecular weight is 451.62 g/mol. The structural formula is as follows:
Cabergoline, USP is a white or almost white crystalline powder practically insoluble in water and hexane, very soluble in ethanol, chloroform, acetone, dichloromethane, soluble in 0.1M HCl and freely soluble in toluene.
Cabergoline tablets, USP for oral administration, contain 0.5 mg of cabergoline, USP. Inactive ingredients consist of citric acid anhydrous powder, croscarmellose sodium, magnesium stearate, and microcrystalline cellulose.