Calcipotriene - Calcipotriene solution
(Calcipotriene)Calcipotriene - Calcipotriene solution Prescribing Information
Calcipotriene Topical Solution, 0.005% (Scalp Solution) is indicated for the topical treatment of chronic, moderately severe psoriasis of the scalp. The safety and effectiveness of topical calcipotriene in dermatoses other than psoriasis have not been established.
Comb the hair to remove scaly debris and after suitably parting, apply Calcipotriene Topical Solution, 0.005% (Scalp Solution), twice daily, only to the lesions, and rub in gently and completely, taking care to prevent the solution spreading onto the forehead. The safety and efficacy of Calcipotriene Topical Solution, 0.005% (Scalp Solution), have been demonstrated in patients treated for eight weeks.
Calcipotriene Topical Solution, 0.005% (Scalp Solution), is contraindicated in those patients with acute psoriatic eruptions or a history of hypersensitivity to any of the components of the preparation. It should not be used by patients with demonstrated hypercalcemia or evidence of vitamin D toxicity.
In controlled clinical trials, the most frequent adverse reactions reported to be related to Calcipotriene Topical Solution, 0.005% (Scalp Solution), use were transient burning, stinging and tingling, which occurred in approximately 23% of patients. Rash was reported in about 11% of patients.
Dry skin, irritation and worsening of psoriasis were reported in 1-5% of patients. Skin atrophy, hyperpigmentation, hypercalcemia, and folliculitis were not observed in these studies, but cannot be excluded.
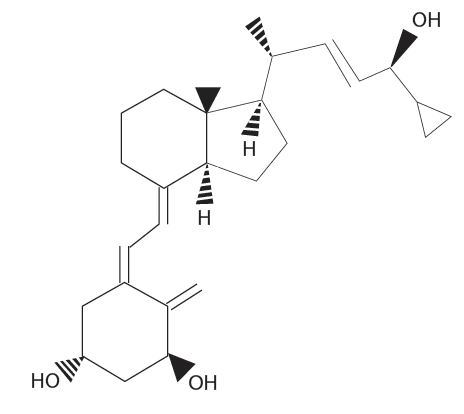
Calcipotriene Topical Solution, 0.005% (Scalp Solution) is a colorless topical solution containing 0.005% calcipotriene in a vehicle of isopropanol (51% v/v), propylene glycol, hydroxypropyl cellulose, sodium citrate, menthol and purified water.
The chemical name of calcipotriene is (5Z,7E,22E,24S)-24-cyclopropyl-9,10-secochola-5, 7,10(19),22-tetraene-1α,3β,24-triol, with the empirical formula C
27H
40O
3, a molecular weight of 412.6, and the following structural formula:
In humans, the natural supply of vitamin D depends mainly on exposure to the ultraviolet rays of the sun for conversion of 7-dehydrocholesterol to vitamin D
3 (cholecalciferol) in the skin. Calcipotriene is a synthetic analog of vitamin D
3.
Although the precise mechanism of calcipotriene’s antipsoriatic action is not fully understood,
Clinical studies with radiolabelled calcipotriene solution indicate that less than 1% of the applied dose of calcipotriene is absorbed through the scalp when the solution (2.0 mL) is applied topically to normal skin or psoriasis plaques (160 cm
2) for 12 hours, and that much of the absorbed calcipotriene is converted to inactive metabolites within 24 hours of application.
Vitamin D and its metabolites are transported in the blood, bound to specific plasma proteins. The active form of the vitamin, 1,25-dihydroxy vitamin D
3 (calcitriol), is known to be recycled via the liver and excreted in the bile. Calcipotriene metabolism following systemic uptake is rapid, and occurs via a similar pathway to the natural hormone. The primary metabolites are much less potent than the parent compound.
There is evidence that maternal 1,25-dihydroxy vitamin D
3 (calcitriol) may enter the fetal circulation, but it is not known whether it is excreted in human milk. The systemic disposition of calcipotriene is expected to be similar to that of the naturally occurring vitamin.