Calcitriol
Calcitriol Prescribing Information
Calcitriol capsule is indicated in the management of secondary hyperparathyroidism and resultant metabolic bone disease in patients with moderate to severe chronic renal failure (Ccr 15 to 55 mL/min) not yet on dialysis. In children, the creatinine clearance value must be corrected for a surface area of 1.73 square meters. A serum iPTH level of ≥ 100 pg/mL is strongly suggestive of secondary hyperparathyroidism.
Calcitriol capsule is indicated in the management of hypocalcemia and the resultant metabolic bone disease in patients undergoing chronic renal dialysis. In these patients, calcitriol administration enhances calcium absorption, reduces serum alkaline phosphatase levels, and may reduce elevated parathyroid hormone levels and the histological manifestations of osteitis fibrosa cystica and defective mineralization.
Calcitriol capsule is also indicated in the management of hypocalcemia and its clinical manifestations in patients with postsurgical hypoparathyroidism, idiopathic hypoparathyroidism, and pseudohypopara-thyroidism.
The optimal daily dose of calcitriol capsules must be carefully determined for each patient. Calcitriol capsule can be administered orally as a capsule (0.25 mcg or 0.50 mcg). Calcitriol capsule therapy should always be started at the lowest possible dose and should not be increased without careful monitoring of serum calcium.
The effectiveness of calcitriol capsule therapy is predicated on the assumption that each patient is receiving an adequate but not excessive daily intake of calcium. Patients are advised to have a dietary intake of calcium at a minimum of 600 mg daily. The U.S. RDA for calcium in adults is 800 mg to 1200 mg. To ensure that each patient receives an adequate daily intake of calcium, the physician should either prescribe a calcium supplement or instruct the patient in proper dietary measures.
Because of improved calcium absorption from the gastrointestinal tract, some patients on calcitriol capsule may be maintained on a lower calcium intake. Patients who tend to develop hypercalcemia may require only low doses of calcium or no supplementation at all.
During the titration period of treatment with calcitriol, serum calcium levels should be checked at least twice weekly. When the optimal dosage of calcitriol has been determined, serum calcium levels should be checked every month (or as given below for individual indications). Samples for serum calcium estimation should be taken without a tourniquet.
Calcitriol should not be given to patients with hypercalcemia or evidence of vitamin D toxicity. Use of Calcitriol in patients with known hypersensitivity to Calcitriol (or drugs of the same class) or any of the inactive ingredients is contraindicated.
Since calcitriol is believed to be the active hormone which exerts vitamin D activity in the body, adverse effects are, in general, similar to those encountered with excessive vitamin D intake, ie, hypercalcemia syndrome or calcium intoxication (depending on the severity and duration of hypercalcemia) (see
The early and late signs and symptoms of vitamin D intoxication associated with hypercalcemia include:
In clinical studies on hypoparathyroidism and pseudohypoparathyroidism, hypercalcemia was noted on at least one occasion in about 1 in 3 patients and hypercalciuria in about 1 in 7 patients. Elevated serum creatinine levels were observed in about 1 in 6 patients (approximately one half of whom had normal levels at baseline).
In concurrent hypercalcemia and hyperphosphatemia, soft-tissue calcification may occur; this can be seen radiographically (see
In patients with normal renal function, chronic hypercalcemia may be associated with an increase in serum creatinine (see
Hypersensitivity reactions (pruritus, rash, urticaria, and very rarely severe erythematous skin disorders) may occur in susceptible individuals. One case of erythema multiforme and one case of allergic reaction (swelling of lips and hives all over the body) were confirmed by rechallenge.
Cholestyramine has been reported to reduce intestinal absorption of fat-soluble vitamins; as such it may impair intestinal absorption of calcitriol (see
The coadministration of phenytoin or phenobarbital will not affect plasma concentrations of calcitriol, but may reduce endogenous plasma levels of 25(OH)D3 by accelerating metabolism. Since blood level of calcitriol will be reduced, higher doses of calcitriol may be necessary if these drugs are administered simultaneously.
Thiazides are known to induce hypercalcemia by the reduction of calcium excretion in urine. Some reports have shown that the concomitant administration of thiazides with calcitriol causes hypercalcemia. Therefore, precaution should be taken when coadministration is necessary.
Calcitriol dosage must be determined with care in patients undergoing treatment with digitalis, as hypercalcemia in such patients may precipitate cardiac arrhythmias (see
Ketoconazole may inhibit both synthetic and catabolic enzymes of calcitriol. Reductions in serum endogenous calcitriol concentrations have been observed following the administration of 300 mg/day to 1200 mg/day ketoconazole for a week to healthy men. However, in vivo drug interaction studies of ketoconazole with calcitriol have not been investigated.
A relationship of functional antagonism exists between vitamin D analogues, which promote calcium absorption, and corticosteroids, which inhibit calcium absorption.
Since calcitriol also has an effect on phosphate transport in the intestine, kidneys and bones, the dosage of phosphate-binding agents must be adjusted in accordance with the serum phosphate concentration.
Since calcitriol is the most potent active metabolite of vitamin D3, pharmacological doses of vitamin D and its derivatives should be withheld during treatment with calcitriol to avoid possible additive effects and hypercalcemia (see
Uncontrolled intake of additional calcium-containing preparations should be avoided (see
Magnesium-containing preparations (eg, antacids) may cause hypermagnesemia and should therefore not be taken during therapy with calcitriol by patients on chronic renal dialysis.
Calcitriol is a synthetic vitamin D analog which is active in the regulation of the absorption of calcium from the gastrointestinal tract and its utilization in the body. Calcitriol is available as capsules containing 0.25 mcg or 0.5 mcg calcitriol All dosage forms contain butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) as antioxidants. The capsules contain medium chain triglycerides. Gelatin capsule shells contain glycerin, sorbitol, with the following dye systems: 0.25 mcg FD&C Yellow No. 6, FD&C red No.3 and titanium dioxide; 0.5 mcg- FD&C Yellow No. 6, FD&C red No.3 and titanium dioxide. The imprinting ink contains propylene glycol, shellac, black iron oxide, isopropyl alcohol, N-butyl alcohol and ammonium hydroxide.
Calcitriol is a white, crystalline compound which occurs naturally in humans. It has a calculated molecular weight of 416.65 and is soluble in organic solvents but relatively insoluble in water. Chemically, calcitriol is 9, 10-seco(5Z,7E)-5,7,10(19) cholestatriene-1α, 3β, 25-triol and has the following structural formula:
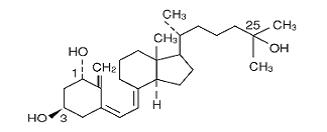
The other names frequently used for calcitriol are lα,25-dihydroxy-cholecalciferol, 1, 25-dihydroxyvitamin D3, 1,25-DHCC, 1,25(OH)2D3 and 1,25-diOHC.