Capecitabine Prescribing Information
Capecitabine Warfarin Interaction: Patients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR or prothrombin time) monitored frequently in order to adjust the anticoagulant dose accordingly. A clinically important Capecitabine-Warfarin drug interaction was demonstrated in a clinical pharmacology trial
Capecitabine can induce diarrhea, sometimes severe. Patients with severe diarrhea should be carefully monitored and given fluid and electrolyte replacement if they become dehydrated. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, the median time to first occurrence of grade 2 to 4 diarrhea was 34 days (range from 1 to 369 days). The median duration of grade 3 to 4 diarrhea was 5 days. National Cancer Institute of Canada (NCIC) grade 2 diarrhea is defined as an increase of 4 to 6 stools/day or nocturnal stools, grade 3 diarrhea as an increase of 7 to 9 stools/day or incontinence and malabsorption, and grade 4 diarrhea as an increase of ≥10 stools/day or grossly bloody diarrhea or the need for parenteral support. If grade 2, 3 or 4 diarrhea occurs, administration of capecitabine should be immediately interrupted until the diarrhea resolves or decreases in intensity to grade 1
Necrotizing enterocolitis (typhlitis) has been reported.
Altered coagulation parameters and/or bleeding have been reported in patients taking capecitabine concomitantly with coumarin-derivative anticoagulants such as warfarin and phenprocoumon
The level of phenytoin should be carefully monitored in patients taking capecitabine and phenytoin dose may need to be reduced
The concentration of 5-fluorouracil is increased and its toxicity may be enhanced by leucovorin. Deaths from severe enterocolitis, diarrhea, and dehydration have been reported in elderly patients receiving weekly leucovorin and fluorouracil.
Other than warfarin, no formal drug-drug interaction studies between capecitabine and other CYP2C9 substrates have been conducted. Care should be exercised when capecitabine is coadministered with CYP2C9 substrates.
Concomitant use with allopurinol may decrease concentration of capecitabine’s active metabolites
Capecitabine is a nucleoside metabolic inhibitor with antineoplastic activity indicated for:
- Adjuvant Colon Cancer()
1.1 Colorectal Cancer- Capecitabine tablets, USP are indicated as a single agent for adjuvant treatment in patients with Dukes' C colon cancer who have undergone complete resection of the primary tumor when treatment with fluoropyrimidine therapy alone is preferred. Capecitabine was non-inferior to 5-fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Physicians should consider results of combination chemotherapy trials, which have shown improvement in DFS and OS, when prescribing single-agent capecitabine in the adjuvant treatment of Dukes' C colon cancer.
- Capecitabine tablets, USP are indicated as first-line treatment of patients with metastatic colorectal carcinoma when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV has not been demonstrated with capecitabine monotherapy. Use of capecitabine tablets, USP instead of 5-FU/LV in combinations has not been adequately studied to assure safety or preservation of the survival advantage.
- Patients with Dukes' C colon cancer
- Metastatic Colorectal Cancer()
1.1 Colorectal Cancer- Capecitabine tablets, USP are indicated as a single agent for adjuvant treatment in patients with Dukes' C colon cancer who have undergone complete resection of the primary tumor when treatment with fluoropyrimidine therapy alone is preferred. Capecitabine was non-inferior to 5-fluorouracil and leucovorin (5-FU/LV) for disease-free survival (DFS). Physicians should consider results of combination chemotherapy trials, which have shown improvement in DFS and OS, when prescribing single-agent capecitabine in the adjuvant treatment of Dukes' C colon cancer.
- Capecitabine tablets, USP are indicated as first-line treatment of patients with metastatic colorectal carcinoma when treatment with fluoropyrimidine therapy alone is preferred. Combination chemotherapy has shown a survival benefit compared to 5-FU/LV alone. A survival benefit over 5-FU/LV has not been demonstrated with capecitabine monotherapy. Use of capecitabine tablets, USP instead of 5-FU/LV in combinations has not been adequately studied to assure safety or preservation of the survival advantage.
- First-line as monotherapy when treatment with fluoropyrimidine therapy alone is preferred
- Metastatic Breast Cancer()
1.2 Breast Cancer- Capecitabine tablets, USP in combination with docetaxel are indicated for the treatment of patients with metastatic breast cancer after failure of prior anthracycline-containing chemotherapy.
- Capecitabine tablets, USP monotherapy is also indicated for the treatment of patients with metastatic breast cancer resistant to both paclitaxel and an anthracycline-containing chemotherapy regimen or resistant to paclitaxel and for whom further anthracycline therapy is not indicated (e.g., patients who have received cumulative doses of 400 mg/m2of doxorubicin or doxorubicin equivalents). Resistance is defined as progressive disease while on treatment, with or without an initial response, or relapse within 6 months of completing treatment with an anthracycline-containing adjuvant regimen.
- In combination with docetaxel after failure of prior anthracycline-containing therapy
- As monotherapy in patients resistant to both paclitaxel and an anthracycline-containing regimen
Capecitabine tablets, USP are supplied as biconvex, oblong film-coated tablets for oral administration. Each light peach-colored tablet contains 150 mg of capecitabine and each peach-colored tablet contains 500 mg of capecitabine.
- Lactation: Advise women not to breastfeed.. ()
8.2 LactationRisk SummaryThere is no information regarding the presence of capecitabine in human milk, or on its effects on milk production or the breast-fed infant. Capecitabine metabolites were present in the milk of lactating mice
[see Data].Because of the potential for serious adverse reactions from capecitabine exposure in breast-fed infants, advise women not to breastfeed during treatment with capecitabine and for 2 weeks after the final dose. - Females and Males of Reproductive Potential:Verify pregnancy status of females prior to initiation of capecitabine. Advise males with female partners of reproductive potential to use effective contraception. ()
8.3 Females and Males of Reproductive PotentialPregnancy TestingPregnancy testing is recommended for females of reproductive potential prior to initiating capecitabine.
ContraceptionFemalesCapecitabine can cause fetal harm when administered to a pregnant woman
[see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the final dose of capecitabine.MalesBased on genetic toxicity findings, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months following the final dose of capecitabine
[see Nonclinical Toxicology (13.1)].InfertilityBased on animal studies, capecitabine may impair fertility in females and males of reproductive potential
[see Nonclinical Toxicology (13.1)]. - Geriatric: Greater incidence of adverse reactions. Monitoring required. ()
8.5 Geriatric UsePhysicians should pay particular attention to monitoring the adverse effects of capecitabine in the elderly
[see Warnings and Precautions (5.10)]. - Hepatic Impairment: Monitoring is recommended in patients with mild to moderate hepatic impairment. ()
8.6 Hepatic InsufficiencyExercise caution when patients with mild to moderate hepatic dysfunction due to liver metastases are treated with capecitabine. The effect of severe hepatic dysfunction on capecitabine is not known
[see Warnings and Precautions (5.11)and Clinical Pharmacology (12.3)]. - Renal Impairment: Reduce capecitabine starting dose in patients with moderate renal impairment (,
2.4 Adjustment of Starting Dose in Special PopulationsRenal ImpairmentNo adjustment to the starting dose of capecitabine tablets are recommended in patients with mild renal impairment (creatinine clearance = 51 to 80 mL/min [Cockroft and Gault, as shown below]). In patients with moderate renal impairment (baseline creatinine clearance = 30 to 50 mL/min), a dose reduction to 75% of the capecitabine tablets starting dose when used as monotherapy or in combination with docetaxel (from 1250 mg/m2to 950 mg/m2twice daily) is recommended
[see Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)]. Subsequent dose adjustment is recommended as outlined inTable 2andTable 3(depending on the regimen) if a patient develops a grade 2 to 4 adverse event[see Warnings and Precautions (5.5)]. The starting dose adjustment recommendations for patients with moderate renal impairment apply to both capecitabine tablets monotherapy and capecitabine tablets in combination use with docetaxel.Cockroft and Gault Equation: (140 - age [yrs]) (body wt [kg]) Creatinine clearance for males = ————————————— (72) (serum creatinine [mg/dL]) Creatinine clearance for females = 0.85 × male value GeriatricsPhysicians should exercise caution in monitoring the effects of capecitabine tablets in the elderly. Insufficient data are available to provide a dosage recommendation.
,8.7 Renal InsufficiencyPatients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed higher exposure for capecitabine, 5-DFUR, and FBAL than in those with normal renal function
[see Contraindications (4.2), Warnings and Precautions (5.5), Dosage and Administration (2.4), and Clinical Pharmacology (12.3)].)12.3 PharmacokineticsAbsorptionFollowing oral administration of 1255 mg/m2BID to cancer patients, capecitabine reached peak blood levels in about 1.5 hours (Tmax) with peak 5-FU levels occurring slightly later, at 2 hours. Food reduced both the rate and extent of absorption of capecitabine with mean Cmaxand AUC0-∞decreased by 60% and 35%, respectively. The Cmaxand AUC0-∞of 5-FU were also reduced by food by 43% and 21%, respectively. Food delayed Tmaxof both parent and 5-FU by 1.5 hours
[see Warnings and Precautions (5), Dosage and Administration (2), and Drug-Food Interaction (7.2)].The pharmacokinetics of capecitabine and its metabolites have been evaluated in about 200 cancer patients over a dosage range of 500 to 3500 mg/m2/day. Over this range, the pharmacokinetics of capecitabine and its metabolite, 5'-DFCR were dose proportional and did not change over time. The increases in the AUCs of 5'-DFUR and 5-FU, however, were greater than proportional to the increase in dose and the AUC of 5-FU was 34% higher on day 14 than on day 1. The interpatient variability in the Cmaxand AUC of 5-FU was greater than 85%.
DistributionPlasma protein binding of capecitabine and its metabolites is less than 60% and is not concentration-dependent. Capecitabine was primarily bound to human albumin (approximately 35%). Capecitabine has a low potential for pharmacokinetic interactions related to plasma protein binding.
Bioactivation and MetabolismCapecitabine is extensively metabolized enzymatically to 5-FU. In the liver, a 60 kDa carboxylesterase hydrolyzes much of the compound to 5'-deoxy-5-fluorocytidine (5'-DFCR). Cytidine deaminase, an enzyme found in most tissues, including tumors, subsequently converts 5'-DFCR to 5'-DFUR. The enzyme, thymidine phosphorylase (dThdPase), then hydrolyzes 5'-DFUR to the active drug 5-FU. Many tissues throughout the body express thymidine phosphorylase. Some human carcinomas express this enzyme in higher concentrations than surrounding normal tissues. Following oral administration of capecitabine 7 days before surgery in patients with colorectal cancer, the median ratio of 5-FU concentration in colorectal tumors to adjacent tissues was 2.9 (range from 0.9 to 8.0). These ratios have not been evaluated in breast cancer patients or compared to 5-FU infusion.
Metabolic Pathway of capecitabine to 5-FU 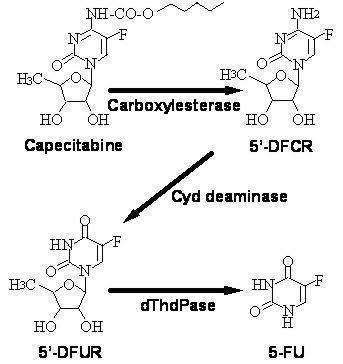
The enzyme dihydropyrimidine dehydrogenase hydrogenates 5-FU, the product of capecitabine metabolism, to the much less toxic 5-fluoro-5, 6-dihydro-fluorouracil (FUH2). Dihydropyrimidinase cleaves the pyrimidine ring to yield 5-fluoro-ureido-propionic acid (FUPA). Finally, β-ureido-propionase cleaves FUPA to α-fluoro-β-alanine (FBAL) which is cleared in the urine.
In vitro enzymatic studies with human liver microsomes indicated that capecitabine and its metabolites (5'-DFUR, 5'-DFCR, 5-FU, and FBAL) did not inhibit the metabolism of test substrates by cytochrome P450 isoenzymes 1A2, 2A6, 3A4, 2C19, 2D6, and 2E1.

Chemical Structure ExcretionCapecitabine and its metabolites are predominantly excreted in urine; 95.5% of administered capecitabine dose is recovered in urine. Fecal excretion is minimal (2.6%). The major metabolite excreted in urine is FBAL which represents 57% of the administered dose. About 3% of the administered dose is excreted in urine as unchanged drug. The elimination half-life of both parent capecitabine and 5-FU was about 0.75 hour.
Effect of Age, Gender, and Race on the Pharmacokinetics of CapecitabineA population analysis of pooled data from the two large controlled studies in patients with metastatic colorectal cancer (n=505) who were administered capecitabine at 1250 mg/m2twice a day indicated that gender (202 females and 303 males) and race (455 white/Caucasian patients, 22 black patients, and 28 patients of other race) have no influence on the pharmacokinetics of 5'-DFUR, 5-FU and FBAL. Age has no significant influence on the pharmacokinetics of 5'-DFUR and 5-FU over the range of 27 to 86 years. A 20% increase in age results in a 15% increase in AUC of FBAL
[see Warnings and Precautions (5.11)and Dosage and Administration (2.4)].Following oral administration of 825 mg/m2capecitabine twice daily for 14 days, Japanese patients (n=18) had about 36% lower Cmaxand 24% lower AUC for capecitabine than the Caucasian patients (n=22). Japanese patients had also about 25% lower Cmaxand 34% lower AUC for FBAL than the Caucasian patients. The clinical significance of these differences is unknown. No significant differences occurred in the exposure to other metabolites (5'-DFCR, 5'-DFUR, and 5-FU).
Effect of Hepatic InsufficiencyCapecitabine has been evaluated in 13 patients with mild to moderate hepatic dysfunction due to liver metastases defined by a composite score including bilirubin, AST/ALT and alkaline phosphatase following a single 1255 mg/m2dose of capecitabine. Both AUC0-∞and Cmaxof capecitabine increased by 60% in patients with hepatic dysfunction compared to patients with normal hepatic function (n=14). The AUC0-∞and Cmaxof 5-FU were not affected. In patients with mild to moderate hepatic dysfunction due to liver metastases, caution should be exercised when capecitabine is administered. The effect of severe hepatic dysfunction on capecitabine is not known
[see Warnings and Precautions (5.11)and Use in Special Populations (8.6)].Effect of Renal InsufficiencyFollowing oral administration of 1250 mg/m2capecitabine twice a day to cancer patients with varying degrees of renal impairment, patients with moderate (creatinine clearance = 30 to 50 mL/min) and severe (creatinine clearance <30 mL/min) renal impairment showed 85% and 258% higher systemic exposure to FBAL on day 1 compared to normal renal function patients (creatinine clearance >80 mL/min). Systemic exposure to 5'-DFUR was 42% and 71% greater in moderately and severely renal impaired patients, respectively, than in normal patients. Systemic exposure to capecitabine was about 25% greater in both moderately and severely renal impaired patients
[see Dosage and Administration (2.4), Contraindications (4.2), Warnings and Precautions (5.5), and Use in Special Populations (8.7)].Effect of Capecitabine on the Pharmacokinetics of WarfarinIn four patients with cancer, chronic administration of capecitabine (1250 mg/m2bid) with a single 20 mg dose of warfarin increased the mean AUC of S-warfarin by 57% and decreased its clearance by 37%. Baseline corrected AUC of INR in these 4 patients increased by 2.8-fold, and the maximum observed mean INR value was increased by 91%
[see Boxed Warningand Drug Interactions (7.1)].Effect of Antacids on the Pharmacokinetics of CapecitabineWhen Maalox® (20 mL), an aluminum hydroxide- and magnesium hydroxide-containing antacid, was administered immediately after capecitabine (1250 mg/m2, n=12 cancer patients), AUC and Cmaxincreased by 16% and 35%, respectively, for capecitabine and by 18% and 22%, respectively, for 5'-DFCR. No effect was observed on the other three major metabolites (5'-DFUR, 5-FU, FBAL) of capecitabine.
Effect of Allopurinol on CapecitabinePublished literature reported that concomitant use with allopurinol may decrease conversion of capecitabine to the active metabolites, FdUMP and FUTP; however, the clinical significance was not fully characterized.
Effect of Capecitabine on the Pharmacokinetics of Docetaxel and Vice VersaA Phase 1 study evaluated the effect of capecitabine on the pharmacokinetics of docetaxel (Taxotere®) and the effect of docetaxel on the pharmacokinetics of capecitabine was conducted in 26 patients with solid tumors. Capecitabine was found to have no effect on the pharmacokinetics of docetaxel (Cmaxand AUC) and docetaxel has no effect on the pharmacokinetics of capecitabine and the 5-FU precursor 5'-DFUR.
- Severe Renal Impairment (
4.1) - Hypersensitivity ()
4.1 Severe Renal ImpairmentCapecitabine is contraindicated in patients with severe renal impairment (creatinine clearance below 30 mL/min [Cockroft and Gault])
[see Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)].
- Coagulopathy:: May result in bleeding, death. Monitor anticoagulant response (e.g., INR) and adjust anticoagulant dose accordingly.()
5.1 CoagulopathyPatients receiving concomitant capecitabine and oral coumarin-derivative anticoagulant therapy should have their anticoagulant response (INR or prothrombin time) monitored closely with great frequency and the anticoagulant dose should be adjusted accordingly
[see Boxed Warning and Drug Interactions (7.1)] - Diarrhea: May be severe. Interrupt capecitabine treatment immediately until diarrhea resolves or decreases to grade 1. Recommend standard antidiarrheal treatments. ()
5.2 DiarrheaCapecitabine can induce diarrhea, sometimes severe. Patients with severe diarrhea should be carefully monitored and given fluid and electrolyte replacement if they become dehydrated. In 875 patients with either metastatic breast or colorectal cancer who received capecitabine monotherapy, the median time to first occurrence of grade 2 to 4 diarrhea was 34 days (range from 1 to 369 days). The median duration of grade 3 to 4 diarrhea was 5 days. National Cancer Institute of Canada (NCIC) grade 2 diarrhea is defined as an increase of 4 to 6 stools/day or nocturnal stools, grade 3 diarrhea as an increase of 7 to 9 stools/day or incontinence and malabsorption, and grade 4 diarrhea as an increase of ≥10 stools/day or grossly bloody diarrhea or the need for parenteral support. If grade 2, 3 or 4 diarrhea occurs, administration of capecitabine should be immediately interrupted until the diarrhea resolves or decreases in intensity to grade 1
[see Dosage and Administration (2.3)]. Standard antidiarrheal treatments (e.g., loperamide) are recommended.Necrotizing enterocolitis (typhlitis) has been reported.
- Cardiotoxicity: Common in patients with a prior history of coronary artery disease. ()
5.3 CardiotoxicityThe cardiotoxicity observed with capecitabine includes myocardial infarction/ischemia, angina, dysrhythmias, cardiac arrest, cardiac failure, sudden death, electrocardiographic changes, and cardiomyopathy. These adverse reactions may be more common in patients with a prior history of coronary artery disease.
- Increased Risk of Severe or Fatal Adverse Reactions in Patients with Low or Absent Dihydropyrimidine Dehydrogenase (DPD) Activity:Withhold or permanently discontinue capecitabine in patients with evidence of acute early-onset or unusually severe toxicity, which may indicate near complete or total absence of DPD activity. No capecitabine dose has been proven safe in patients with absent DPD activity. ()
5.4 Dihydropyrimidine Dehydrogenase DeficiencyBased on postmarketing reports, patients with certain homozygous or certain compound heterozygous mutations in the DPD gene that result in complete or near complete absence of DPD activity are at increased risk for acute early-onset of toxicity and severe, life-threatening, or fatal adverse reactions caused by capecitabine (e.g., mucositis, diarrhea, neutropenia, and neurotoxicity). Patients with partial DPD activity may also have increased risk of severe, life-threatening, or fatal adverse reactions caused by capecitabine.
Withhold or permanently discontinue capecitabine based on clinical assessment of the onset, duration and severity of the observed toxicities in patients with evidence of acute early-onset or unusually severe toxicity, which may indicate near complete or total absence of DPD activity. No capecitabine dose has been proven safe for patients with complete absence of DPD activity. There is insufficient data to recommend a specific dose in patients with partial DPD activity as measured by any specific test.
- Dehydration and Renal Failure: Interrupt capecitabine treatment until dehydration is corrected. Potential risk of acute renal failure secondary to dehydration. Monitor and correct dehydration. ()
5.5 Dehydration and Renal FailureDehydration has been observed and may cause acute renal failure which can be fatal. Patients with pre-existing compromised renal function or who are receiving concomitant capecitabine with known nephrotoxic agents are at higher risk. Patients with anorexia, asthenia, nausea, vomiting or diarrhea may rapidly become dehydrated. Monitor patients when capecitabine is administered to prevent and correct dehydration at the onset. If grade 2 (or higher) dehydration occurs, capecitabine treatment should be immediately interrupted and the dehydration corrected. Treatment should not be restarted until the patient is rehydrated and any precipitating causes have been corrected or controlled. Dose modifications should be applied for the precipitating adverse event as necessary
[see Dosage and Administration (2.3)].Patients with moderate renal impairment at baseline require dose reduction
[see Dosage and Administration (2.4)]. Patients with mild and moderate renal impairment at baseline should be carefully monitored for adverse reactions. Prompt interruption of therapy with subsequent dose adjustments is recommended if a patient develops a grade 2 to 4 adverse event as outlined inTable 2[see Dosage and Administration (2.3), Use in Specific Populations (8.7), and Clinical Pharmacology (12.3)]. - Embryo-Fetal Toxicity:Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and to use effective contraception. (,
5.6 Embryo-Fetal ToxicityBased on findings from animal reproduction studies and its mechanism of action, capecitabine may cause fetal harm when given to a pregnant woman
[see Clinical Pharmacology (12.1)]. Limited available data are not sufficient to inform use of capecitabine in pregnant women. In animal reproduction studies, administration of capecitabine to pregnant animals during the period of organogenesis caused embryolethality and teratogenicity in mice and embryolethality in monkeys at 0.2 and 0.6 times the exposure (AUC) in patients receiving the recommended dose respectively[see Use in Specific Populations (8.1)]. Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the last dose of capecitabine[see Use in Specific Populations (8.3)].,8.1 PregnancyRisk SummaryBased on findings in animal reproduction studies and its mechanism of action, capecitabine can cause fetal harm when administered to a pregnant woman
[see Clinical Pharmacology (12.1)].Limited available human data are not sufficient to inform the drug-associated risk during pregnancy. In animal reproduction studies, administration of capecitabine to pregnant animals during the period of organogenesis caused embryo lethality and teratogenicity in mice and embryo lethality in monkeys at 0.2 and 0.6 times the exposure (AUC) in patients receiving the recommended dose respectively[see Data].Apprise pregnant women of the potential risk to a fetus.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
DataAnimal Data
Oral administration of capecitabine to pregnant mice during the period of organogenesis at a dose of 198 mg/kg/day caused malformations and embryo lethality. In separate pharmacokinetic studies, this dose in mice produced 5’-DFUR AUC values that were approximately 0.2 times the AUC values in patients administered the recommended daily dose. Malformations in mice included cleft palate, anophthalmia, microphthalmia, oligodactyly, polydactyly, syndactyly, kinky tail and dilation of cerebral ventricles. Oral administration of capecitabine to pregnant monkeys during the period of organogenesis at a dose of 90 mg/kg/day, caused fetal lethality. This dose produced 5’-DFUR AUC values that were approximately 0.6 times the AUC values in patients administered the recommended daily dose.)8.3 Females and Males of Reproductive PotentialPregnancy TestingPregnancy testing is recommended for females of reproductive potential prior to initiating capecitabine.
ContraceptionFemalesCapecitabine can cause fetal harm when administered to a pregnant woman
[see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment and for 6 months following the final dose of capecitabine.MalesBased on genetic toxicity findings, advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months following the final dose of capecitabine
[see Nonclinical Toxicology (13.1)].InfertilityBased on animal studies, capecitabine may impair fertility in females and males of reproductive potential
[see Nonclinical Toxicology (13.1)]. - Mucocutaneous and Dermatologic Toxicity: Severe mucocutaneous reactions, Steven-Johnson Syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), have been reported. Capecitabine should be permanently discontinued in patients who experience a severe mucocutaneous reaction during treatment. Capecitabine may induce hand-and-foot syndrome. Persistent or severe hand-and-foot syndrome can lead to loss of fingerprints which could impact patient identification. Interrupt capecitabine treatment until the hand-and-foot syndrome event resolves or decreases in intensity.()
5.7 Mucocutaneous and Dermatologic ToxicitySevere mucocutaneous reactions, some with fatal outcome, such as Stevens-Johnson syndrome and Toxic Epidermal Necrolysis (TEN) can occur in patients treated with capecitabine
[see Adverse Reactions (6.4)]. Capecitabine should be permanently discontinued in patients who experience a severe mucocutaneous reaction possibly attributable to capecitabine treatment.Hand-and-foot syndrome (palmar-plantar erythrodysesthesia or chemotherapy-induced acral erythema) is a cutaneous toxicity. Median time to onset was 79 days (range from 11 to 360 days) with a severity range of grades 1 to 3 for patients receiving capecitabine monotherapy in the metastatic setting. Grade 1 is characterized by any of the following: numbness, dysesthesia/paresthesia, tingling, painless swelling or erythema of the hands and/or feet and/or discomfort which does not disrupt normal activities. Grade 2 hand-and-foot syndrome is defined as painful erythema and swelling of the hands and/or feet and/or discomfort affecting the patient’s activities of daily living. Grade 3 hand-and-foot syndrome is defined as moist desquamation, ulceration, blistering or severe pain of the hands and/or feet and/or severe discomfort that causes the patient to be unable to work or perform activities of daily living. Persistent or severe hand-and-foot syndrome (grade 2 and above) can eventually lead to loss of fingerprints which could impact patient identification. If grade 2 or 3 hand-and-foot syndrome occurs, administration of capecitabine should be interrupted until the event resolves or decreases in intensity to grade 1. Following grade 3 hand-and-foot syndrome, subsequent doses of capecitabine should be decreased
[see Dosage and Administration (2.3)]. - Hyperbilirubinemia: Interrupt capecitabine treatment immediately until the hyperbilirubinemia resolves or decreases in intensity. ()
5.8 HyperbilirubinemiaIn 875 patients with either metastatic breast or colorectal cancer who received at least one dose of capecitabine 1250 mg/m2twice daily as monotherapy for 2 weeks followed by a 1-week rest period, grade 3 (1.5 to 3 × ULN) hyperbilirubinemia occurred in 15.2% (n=133) of patients and grade 4 (>3 × ULN) hyperbilirubinemia occurred in 3.9% (n=34) of patients. Of 566 patients who had hepatic metastases at baseline and 309 patients without hepatic metastases at baseline, grade 3 or 4 hyperbilirubinemia occurred in 22.8% and 12.3%, respectively. Of the 167 patients with grade 3 or 4 hyperbilirubinemia, 18.6% (n=31) also had postbaseline elevations (grades 1 to 4, without elevations at baseline) in alkaline phosphatase and 27.5% (n=46) had postbaseline elevations in transaminases at any time (not necessarily concurrent). The majority of these patients, 64.5% (n=20) and 71.7% (n=33), had liver metastases at baseline. In addition, 57.5% (n=96) and 35.3% (n=59) of the 167 patients had elevations (grades 1 to 4) at both prebaseline and postbaseline in alkaline phosphatase or transaminases, respectively. Only 7.8% (n=13) and 3.0% (n=5) had grade 3 or 4 elevations in alkaline phosphatase or transaminases.
In the 596 patients treated with capecitabine as first-line therapy for metastatic colorectal cancer, the incidence of grade 3 or 4 hyperbilirubinemia was similar to the overall clinical trial safety database of capecitabine monotherapy. The median time to onset for grade 3 or 4 hyperbilirubinemia in the colorectal cancer population was 64 days and median total bilirubin increased from 8 µm/L at baseline to 13 µm/L during treatment with capecitabine. Of the 136 colorectal cancer patients with grade 3 or 4 hyperbilirubinemia, 49 patients had grade 3 or 4 hyperbilirubinemia as their last measured value, of which 46 had liver metastases at baseline.
In 251 patients with metastatic breast cancer who received a combination of capecitabine and docetaxel, grade 3 (1.5 to 3 × ULN) hyperbilirubinemia occurred in 7% (n=17) and grade 4 (>3 × ULN) hyperbilirubinemia occurred in 2% (n=5).
If drug-related grade 3 to 4 elevations in bilirubin occur, administration of capecitabine should be immediately interrupted until the hyperbilirubinemia decreases to ≤3.0 X ULN
[see recommended dose modifications underDosage and Administration (2.3)]. - Hematologic: Do not treat patients with neutrophil counts <1.5 × 10
9/L or thrombocyte counts <100 × 10
9/L. If grade 3 to 4 neutropenia or thrombocytopenia occurs, stop therapy until condition resolves. ()5.9 HematologicIn 875 patients with either metastatic breast or colorectal cancer who received a dose of 1250 mg/m2administered twice daily as monotherapy for 2 weeks followed by a 1-week rest period, 3.2%, 1.7%, and 2.4% of patients had grade 3 or 4 neutropenia, thrombocytopenia or decreases in hemoglobin, respectively. In 251 patients with metastatic breast cancer who received a dose of capecitabine in combination with docetaxel, 68% had grade 3 or 4 neutropenia, 2.8% had grade 3 or 4 thrombocytopenia, and 9.6% had grade 3 or 4 anemia.
Patients with baseline neutrophil counts of <1.5 × 109/L and/or thrombocyte counts of <100 × 109/L should not be treated with capecitabine. If unscheduled laboratory assessments during a treatment cycle show grade 3 or 4 hematologic toxicity, treatment with capecitabine should be interrupted.