Carbidopa And Levodopa
Carbidopa And Levodopa Prescribing Information
Carbidopa and levodopa orally disintegrating tablets are indicated in the treatment of Parkinson’s disease, post-encephalitic parkinsonism, and symptomatic parkinsonism that may follow carbon monoxide intoxication or manganese intoxication.
Carbidopa allows patients treated for Parkinson’s disease to use much lower doses of levodopa. Some patients who responded poorly to levodopa have improved on carbidopa and levodopa orally disintegrating tablets. This is most likely due to decreased peripheral decarboxylation of levodopa caused by administration of carbidopa rather than by a primary effect of carbidopa on the nervous system. Carbidopa has not been shown to enhance the intrinsic efficacy of levodopa.
Carbidopa may also reduce nausea and vomiting and permit more rapid titration of levodopa.
Just prior to administration, GENTLY remove the tablet from the bottle with dry hands. IMMEDIATELY place the carbidopa and levodopa orally disintegrating tablet on top of the tongue where it will dissolve in seconds, then swallow with saliva. Administration with liquid is not necessary.
The optimum daily dosage of carbidopa and levodopa orally disintegrating tablets must be determined by careful titration in each patient. Carbidopa and levodopa orally disintegrating tablets are available in a 1:4 ratio of carbidopa to levodopa (carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg) as well as 1:10 ratio (carbidopa and levodopa orally disintegrating tablets 25 mg/250 mg and carbidopa and levodopa orally disintegrating tablets 10 mg/100 mg). Tablets of the two ratios may be given separately or combined as needed to provide the optimum dosage.
Studies show that peripheral dopa decarboxylase is saturated by carbidopa at approximately 70 to 100 mg a day. Patients receiving less than this amount of carbidopa are more likely to experience nausea and vomiting.
Dosage is best initiated with one tablet of carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg three times a day. This dosage schedule provides 75 mg of carbidopa per day. Dosage may be increased by one tablet every day or every other day, as necessary, until a dosage of eight tablets of carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg a day is reached.
If carbidopa and levodopa orally disintegrating tablet 10 mg/100 mg is used, dosage may be initiated with one tablet three or four times a day. However, this will not provide an adequate amount of carbidopa for many patients. Dosage may be increased by one tablet every day or every other day until a total of eight tablets (2 tablets q.i.d.) is reached.
Therapy should be individualized and adjusted according to the desired therapeutic response. At least 70 to 100 mg of carbidopa per day should be provided. When a greater proportion of carbidopa is required, one tablet of carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg may be substituted for each tablet of carbidopa and levodopa orally disintegrating tablets 10 mg/100 mg. When more levodopa is required, carbidopa and levodopa orally disintegrating tablets 25 mg/250 mg should be substituted for carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg or carbidopa and levodopa orally disintegrating tablets 10 mg/100 mg. If necessary, the dosage of carbidopa and levodopa orally disintegrating tablets 25 mg/250 mg may be increased by one-half or one tablet every day or every other day to a maximum of eight tablets a day. Experience with total daily dosages of carbidopa greater than 200 mg is limited.
Because both therapeutic and adverse responses occur more rapidly with carbidopa and levodopa orally disintegrating tablets than with levodopa alone, patients should be monitored closely during the dose adjustment period. Specifically, involuntary movements will occur more rapidly with carbidopa and levodopa orally disintegrating tablets than with levodopa. The occurrence of involuntary movements may require dosage reduction. Blepharospasm may be a useful early sign of excess dosage in some patients.
Standard drugs for Parkinson’s disease, other than levodopa without a decarboxylase inhibitor, may be used concomitantly while carbidopa and levodopa orally disintegrating tablet is being administered, although dosage adjustments may be required.
Sporadic cases of hyperpyrexia and confusion have been associated with dose reductions and withdrawal of carbidopa and levodopa. Patients should be observed carefully if abrupt reduction or discontinuation of carbidopa and levodopa orally disintegrating tablets is required, especially if the patient is receiving neuroleptics. (See WARNINGS.)
If general anesthesia is required, carbidopa and levodopa orally disintegrating tablets may be continued as long as the patient is permitted to take fluids and medication by mouth. If therapy is interrupted temporarily, the patient should be observed for symptoms resembling NMS, and the usual daily dosage may be administered as soon as the patient is able to take oral medication.
Nonselective monoamine oxidase (MAO) inhibitors are contraindicated for use with carbidopa and levodopa orally disintegrating tablets. These inhibitors must be discontinued at least two weeks prior to initiating therapy with carbidopa and levodopa orally disintegrating tablets. Carbidopa and levodopa orally disintegrating tablets may be administered concomitantly with the manufacturer’s recommended dose of an MAO inhibitor with selectivity for MAO type B (e.g., selegiline HCI) (see PRECAUTIONS,
Carbidopa and levodopa orally disintegrating tablets are contraindicated in patients with known hypersensitivity to any component of this drug, and in patients with narrow-angle glaucoma.
The most common adverse reactions reported with carbidopa and levodopa therapy have included dyskinesias, such as choreiform, dystonic, and other involuntary movements and nausea.
The following other adverse reactions have been reported with carbidopa and levodopa:
Other adverse reactions that have been reported with levodopa alone and with various carbidopa and levodopa formulations, and may occur with carbidopa and levodopa orally disintegrating tablets are:
Symptomatic postural hypotension occurred when carbidopa and levodopa was added to the treatment of a patient receiving antihypertensive drugs. Therefore, when therapy with carbidopa and levodopa orally disintegrating tablets is started, dosage adjustment of the antihypertensive drug may be required.
For patients receiving MAO inhibitors (Type A or B), see CONTRAINDICATIONS. Concomitant therapy with selegiline and carbidopa and levodopa may be associated with severe orthostatic hypotension not attributable to carbidopa and levodopa alone (see CONTRAINDICATIONS).
There have been rare reports of adverse reactions, including hypertension and dyskinesia, resulting from the concomitant use of tricyclic antidepressants and carbidopa and levodopa.
Dopamine D2 receptor antagonists (e.g., phenothiazines, butyrophenones, risperidone) and isoniazid may reduce the therapeutic effects of levodopa. In addition, the beneficial effects of levodopa in Parkinson’s disease have been reported to be reversed by phenytoin and papaverine. Patients taking these drugs with carbidopa and levodopa orally disintegrating tablets should be carefully observed for loss of therapeutic response.
Use of carbidopa and levodopa orally disintegrating tablets with dopamine-depleting agents (e.g., reserpine and tetrabenazine) or other drugs known to deplete monoamine stores is not recommended.
Carbidopa and levodopa orally disintegrating tablets and iron salts or multivitamins containing iron salts should be coadministered with caution. Iron salts can form chelates with levodopa and carbidopa and consequently reduce the bioavailability of carbidopa and levodopa.
Although metoclopramide may increase the bioavailability of levodopa by increasing gastric emptying, metoclopramide may also adversely affect disease control by its dopamine receptor antagonistic properties.
Carbidopa and levodopa orally disintegrating tablet, USP is a combination of carbidopa and levodopa for the treatment of Parkinson’s disease and syndrome. Carbidopa and levodopa orally disintegrating tablet is an orally administered formulation of carbidopa and levodopa which rapidly disintegrates on the tongue and does not require water to aid dissolution or swallowing.
Carbidopa USP, an inhibitor of aromatic amino acid decarboxylation, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 244.24. It is designated chemically as (-)-L-α-hydrazino-α-methyl-β-(3,4-dihydroxybenzene) propanoic acid monohydrate. Its molecular formula is C10H14N2O4
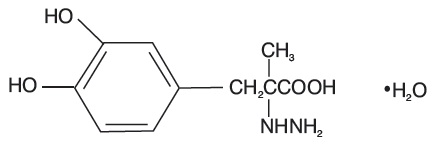
Tablet content is expressed in terms of anhydrous carbidopa which has a molecular weight of 226.23.
Levodopa USP, an aromatic amino acid, is a white, crystalline compound, slightly soluble in water, with a molecular weight of 197.2. It is designated chemically as (—)-L-α-amino-β-(3,4-dihydroxybenzene) propanoic acid. Its molecular formula is C9H11NO4, and its structural formula is:
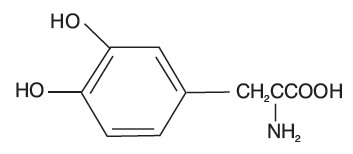
Carbidopa and levodopa orally disintegrating tablets, USP are supplied as tablets in three strengths:
Carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg, containing 25 mg of carbidopa and 100 mg of levodopa.
Carbidopa and levodopa orally disintegrating tablets 10 mg/100 mg, containing 10 mg of carbidopa and 100 mg of levodopa.
Carbidopa and levodopa orally disintegrating tablets 25 mg/250 mg, containing 25 mg of carbidopa and 250 mg of levodopa.
Inactive ingredients are povidone, mannitol, calcium silicate, crospovidone, talc, magnesium stearate, aspartame, tutti-frutti flavor, colloidal silicon dioxide, microcrystalline cellulose. Carbidopa and levodopa orally disintegrating tablets 10 mg/100 mg and 25 mg/250 mg also contain FD&C blue #2 (aluminum lake). Carbidopa and levodopa orally disintegrating tablets 25 mg/100 mg also contain D&C Yellow 10 Aluminum Lake and FD & C Yellow 6 Lake.
Meets USP dissolution test 2.