Carboplatin Prescribing Information
Carboplatin Injection should be administered under the supervision of a qualified physician experienced in the use of cancer chemotherapeutic agents. Appropriate management of therapy and complications is possible only when adequate treatment facilities are readily available.
Bone marrow suppression is dose related and may be severe, resulting in infection and/or bleeding. Anemia may be cumulative and may require transfusion support. Vomiting is another frequent drug-related side effect.
Anaphylactic-like reactions to carboplatin have been reported and may occur within minutes of carboplatin administration. Epinephrine, corticosteroids, and antihistamines have been employed to alleviate symptoms.
Carboplatin Injection is indicated for the palliative treatment of patients with ovarian carcinoma recurrent after prior chemotherapy, including patients who have been previously treated with cisplatin.
Within the group of patients previously treated with cisplatin, those who have developed progressive disease while receiving cisplatin therapy may have a decreased response rate.
Because renal function is often decreased in elderly patients, formula dosing of carboplatin based on estimates of GFR should be used in elderly patients to provide predictable plasma carboplatin AUCs and thereby minimize the risk of toxicity.
Carboplatin Injection is contraindicated in patients with a history of severe allergic reactions to cisplatin or other platinum-containing compounds.
Carboplatin Injection should not be employed in patients with severe bone marrow depression or significant bleeding.
Pain and asthenia were the most frequently reported miscellaneous adverse effects; their relationship to the tumor and to anemia was likely. Alopecia was reported (3%). Cardiovascular, respiratory, genitourinary, and mucosal side effects have occurred in 6% or less of the patients. Cardiovascular events (cardiac failure, embolism, cerebrovascular accidents) were fatal in less than 1% of the patients and did not appear to be related to chemotherapy. Cancer-associated hemolytic uremic syndrome has been reported rarely.
Malaise, anorexia, hypertension, dehydration, and stomatitis have been reported as part of postmarketing surveillance.
Carboplatin Injection is supplied as a sterile, pyrogen-free solution available in 10 mg per mL multiple dose vials containing 50 mg, 150 mg, 450 mg or 600 mg of carboplatin for administration by intravenous infusion. Each mL contains: carboplatin 10 mg, and water for injection to volume.
Carboplatin is a platinum coordination compound. The chemical name for carboplatin is platinum, diammine [1,1-cyclobutane-dicarboxylato(2-)-0,0’]-, (SP-4-2), and has the following structural formula:
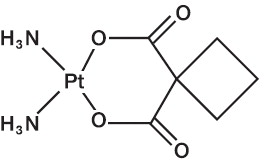
Carboplatin is a crystalline powder. It is soluble in water at a rate of approximately 14 mg/mL, and the pH of a 1% solution is 5 to 7. It is virtually insoluble in ethanol, acetone, and dimethylacetamide.