Carisoprodol
Carisoprodol Prescribing Information
Carisoprodol Tablets, USP are indicated for the relief of discomfort associated with acute, painful musculoskeletal conditions in adults.
Carisoprodol Tablets, USP should only be used for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use has not been established and because acute, painful musculoskeletal conditions are generally of short duration [
The recommended dose of carisoprodol is 250 mg to 350 mg three times a day and at bedtime. The recommended maximum duration of carisoprodol use is up to two or three weeks.
- Recommended dose is 250 mg to 350 mg three times a day and at bedtime.
The recommended dose of carisoprodol is 250 mg to 350 mg three times a day and at bedtime. The recommended maximum duration of carisoprodol use is up to two or three weeks.
- Recommended dose is 250 mg to 350 mg three times a day and at bedtime.
The recommended dose of carisoprodol is 250 mg to 350 mg three times a day and at bedtime. The recommended maximum duration of carisoprodol use is up to two or three weeks.
350 mg Tablets: white, round, unscored tablets debossed “2410 V” on one side and plain on the reverse side.
Carisoprodol tablets are contraindicated in patients with a history of acute intermittent porphyria or a hypersensitivity reaction to a carbamate such as meprobamate.
Carisoprodol Tablets USP are available as 350 mg round, white tablets for oral administration. Carisoprodol is a white, crystalline powder, having a mild, characteristic odor and a bitter taste. It is slightly soluble in water; freely soluble in alcohol, in chloroform, and in acetone; and its solubility is practically independent of pH. Carisoprodol is present as a racemic mixture. Chemically, carisoprodol is N-isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate and the molecular formula is C
12H
24N
2O
4, with a molecular weight of 260.33. The structural formula is:
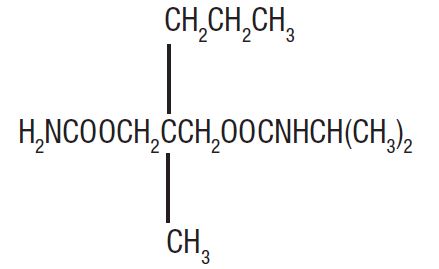
Other ingredients in Carisoprodol Tablets, USP include croscarmellose sodium, hydrogenated vegetable oil, hypromellose, magnesium stearate and microcrystalline cellulose.
The safety and efficacy of carisoprodol for the relief of acute, idiopathic mechanical low back pain was evaluated in two, 7-day, double blind, randomized, multicenter, placebo controlled, U.S. trials (Studies 1 and 2). Patients had to be 18 to 65 years old and had to have acute back pain (≤ 3 days of duration) to be included in the trials. Patients with chronic back pain; at increased risk for vertebral fracture (e.g., history of osteoporosis); with a history of spinal pathology (e.g., herniated nucleus pulposis, spondylolisthesis or spinal stenosis); with inflammatory back pain, or with evidence of a neurologic deficit were excluded from participation. Concomitant use of analgesics (e.g., acetaminophen, NSAIDs, tramadol, opioid agonists), other muscle relaxants, botulinum toxin, sedatives (e.g., barbiturates, benzodiazepines, promethazine hydrochloride), and anti-epileptic drugs was prohibited.
In Study 1, patients were randomized to one of three treatment groups (i.e., carisoprodol 250 mg, carisoprodol 350 mg, or placebo) and in Study 2 patients were randomized to two treatment groups (i.e., carisoprodol 250 mg or placebo). In both studies, patients received study medication three times a day and at bedtime for seven days.
The primary endpoints were the relief from starting backache and the global impression of change, as reported by patients, on Study Day 3. Both endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome) in both studies. The primary statistical comparison was between the carisoprodol 250 mg and placebo groups in both studies.
The proportion of patients who used concomitant acetaminophen, NSAIDs, tramadol, opioid agonists, other muscle relaxants, and benzodiazepines was similar in the treatment groups.
The results for the primary efficacy evaluations in the acute, low back pain studies are presented in Table 3.
a in Studies 1 and 2
Study | Parameter | Placebo | Carisoprodol 250 mg | Carisoprodol 350 mg | |
1 | Number of Patients | n=269 | n=264 | n=273 | |
Relief from Starting Backache, Mean (SE) b | 1.4 (0.1) | 1.8 (0.1) | 1.8 (0.1) | ||
Difference between Carisoprodol and Placebo, Mean (SE) | 0.4 (0.2, 0.5) | 0.4 (0.2, 0.6) | |||
Global Impression of Change, Mean (SE) b | 1.9 (0.1) | 2.2 (0.1) | 2.2 (0.1) | ||
Difference between Carisoprodol and Placebo, Mean (SE) | 0.2 (0.1, 0.4) | 0.3 (0.1, 0.4) | |||
2 | Number of Patients | n=278 | n=269 | ||
Relief from Starting Backache, Mean (SE) b | 1.1 (0.1) | 1.8 (0.1) | |||
Difference between Carisoprodol and Placebo, Mean (SE) | 0.7 (0.5, 0.9) | ||||
Global Impression of Change, Mean (SE) b | 1.7 (0.1) | 2.2 (0.1) | |||
Difference between Carisoprodol and Placebo, Mean (SE) | 0.5 (0.4, 0.7) |
a The primary efficacy endpoints (Relief from Starting Backache and Global Impression of Change) were assessed by the patients on Study Day 3. These endpoints were scored on a 5-point rating scale from 0 (worst outcome) to 4 (best outcome).
b Mean is the least squared mean and SE is the standard error of the mean. The ANOVA model was used for the primary statistical comparison between the carisoprodol 250 mg and placebo groups.
Patients treated with carisoprodol experienced improvement in function as measured by the Roland-Morris Disability Questionnaire (RMDQ) score on Days 3 and 7.