Caspofungin Acetate
Caspofungin Acetate Prescribing Information
Caspofungin acetate for injection is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients. (
Caspofungin acetate for injection is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections.
• Treatment of esophageal candidiasis.
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies.
Caspofungin acetate for injection is indicated as empirical therapy for presumed fungal infections in febrile, neutropenic adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of candidemia and the following candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of esophageal candidiasis in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of invasive aspergillosis in adult and pediatric patients (3 months of age and older) who are refractory to or intolerant of other therapies
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections. (
Caspofungin acetate for injection is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections.
• Treatment of esophageal candidiasis.
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies.
Caspofungin acetate for injection is indicated as empirical therapy for presumed fungal infections in febrile, neutropenic adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of candidemia and the following candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of esophageal candidiasis in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of invasive aspergillosis in adult and pediatric patients (3 months of age and older) who are refractory to or intolerant of other therapies
• Treatment of esophageal candidiasis. (
Caspofungin acetate for injection is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections.
• Treatment of esophageal candidiasis.
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies.
Caspofungin acetate for injection is indicated as empirical therapy for presumed fungal infections in febrile, neutropenic adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of candidemia and the following candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of esophageal candidiasis in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of invasive aspergillosis in adult and pediatric patients (3 months of age and older) who are refractory to or intolerant of other therapies
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies. (
Caspofungin acetate for injection is an echinocandin antifungal indicated in adults and pediatric patients (3 months of age and older) for:
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis and pleural space infections.
• Treatment of esophageal candidiasis.
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies.
Caspofungin acetate for injection is indicated as empirical therapy for presumed fungal infections in febrile, neutropenic adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of candidemia and the following candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of esophageal candidiasis in adult and pediatric patients (3 months of age and older)
Caspofungin acetate for injection is indicated for the treatment of invasive aspergillosis in adult and pediatric patients (3 months of age and older) who are refractory to or intolerant of other therapies
Administer caspofungin acetate for injection by slow intravenous (IV) infusion over approximately 1 hour. Do not administer caspofungin acetate for injection by IV bolus administration.
• Administer by slow intravenous (IV) infusion over approximately 1 hour. Do not administer by IV bolus administration.
• Do not mix or co-infuse caspofungin acetate for injection with other medications. Do not use diluents containing dextrose (α-D-glucose).
The dosage and duration of caspofungin acetate for injection treatment for each indications are as follows:
Administer a single 70 mg loading dose on Day 1, followed by 50 mg once daily thereafter. Duration of treatment should be dictated by the patient's clinical and microbiological response. In general, continue antifungal therapy for at least 14 days after the last positive culture. Patients with neutropenia who remain persistently neutropenic may warrant a longer course of therapy pending resolution of the neutropenia.
• Administer a single 70 mg loading dose on Day 1, followed by 50 mg once daily for all indications except esophageal candidiasis.
• For esophageal candidiasis, use 50 mg once daily with no loading dose.
For all indications, administer a single 70 mg/m2loading dose on Day 1, followed by 50 mg/m2once daily thereafter.
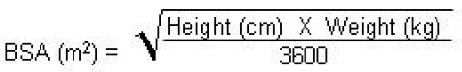
Following calculation of the patient's BSA, the loading dose in milligrams should be calculated as BSA (m2) X 70 mg/m2. The maintenance dose in milligrams should be calculated as BSA (m2) X 50 mg/m2.
Duration of treatment should be individualized to the indication, as described for each indication in adults
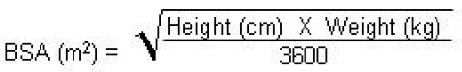
• Dosing should be based on the patient's body surface area.
• For all indications, administer a single 70 mg/m2 loading dose on Day 1, followed by 50 mg/m2 once daily thereafter.
• Maximum loading dose and daily maintenance dose should not exceed 70 mg, regardless of the patient's calculated dose.
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), caspofungin acetate for injection 35 mg once daily is recommended based upon pharmacokinetic data
Reduce dosage for adult patients with moderate hepatic impairment (35 mg once daily, with a 70 mg loading dose on Day 1 where appropriate).
• Use 70 mg once daily dose for adult patients on rifampin.
• Consider dose increase to 70 mg once daily for adult patients on nevirapine, efavirenz, carbamazepine, dexamethasone, or phenytoin.
• Pediatric patients receiving these same concomitant medications may also require an increase in dose to 70 mg/m2 once daily (maximum daily dose not to exceed 70 mg).
Caspofungin Acetate for Injection 50 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with an aluminum seal and a plastic cap. Caspofungin acetate for injection 50 mg vial contains 50 mg of caspofungin equivalent to 55.5 mg of caspofungin acetate.
Caspofungin Acetate for Injection 70 mg is a white to off-white lyophilized cake or powder for reconstitution in a single-dose glass vial with an aluminum seal and a plastic cap. Caspofungin acetate for injection 70 mg vial contains 70 mg of caspofungin equivalent to 77.7 mg of caspofungin acetate.
•
Based on animal data, caspofungin may cause fetal harm
In animal studies, caspofungin caused embryofetal toxicity, including increased resorptions, increased peri-implantation loss, and incomplete ossification at multiple fetal sites when administered intravenously to pregnant rats and rabbits during organogenesis at doses up to 0.8 and 2 times the clinical dose, respectively
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
In animal reproduction studies, pregnant rats dosed intravenously with caspofungin during organogenesis (gestational days [GD] 6 to 20) at 0.5, 2, or 5 mg/kg/day (up to 0.8 times the clinical dose based on body surface area comparison) showed increased resorptions and peri-implantation losses at 5 mg/kg/day. Incomplete ossification of the skull and torso and increased incidences of cervical rib were noted in offspring born to pregnant rats treated at doses up to 5 mg/kg/day. In pregnant rabbits treated with intravenous caspofungin during organogenesis (GD 7 to 20) at doses of 1, 3, or 6 mg/kg/day (approximately 2 times the clinical dose based on body surface area comparison), increased fetal resorptions and increased incidence of incomplete ossification of the talus/calcaneus in offspring were observed at the highest dose tested. Caspofungin crossed the placenta in rats and rabbits and was detectable in fetal plasma.
In peri- and postnatal development study in rats, intravenous caspofungin administered at 0.5, 2 or 5 mg/kg/day from Day 6 of gestation through Day 20 of lactation was not associated with any adverse effects on reproductive performance or subsequent development of first generation (F1) offspring or malformations in second generation (F2) offspring.
•
The safety and effectiveness of caspofungin in pediatric patients 3 months to 17 years of age are supported by evidence from adequate and well-controlled studies in adults, pharmacokinetic data in pediatric patients, and additional data from prospective studies in pediatric patients 3 months to 17 years of age for the following indications
• Empirical therapy for presumed fungal infections in febrile, neutropenic patients.
• Treatment of candidemia and the following Candida infections: intra-abdominal abscesses, peritonitis, and pleural space infections.
• Treatment of esophageal candidiasis.
• Treatment of invasive aspergillosis in patients who are refractory to or intolerant of other therapies (e.g., amphotericin B, lipid formulations of amphotericin B, itraconazole).
The efficacy and safety of caspofungin has not been adequately studied in prospective clinical trials involving neonates and infants under 3 months of age. Although limited pharmacokinetic data were collected in neonates and infants below 3 months of age, these data are insufficient to establish a safe and effective dose of caspofungin in the treatment of neonatal candidiasis. Invasive candidiasis in neonates has a higher rate of CNS and multi-organ involvement than in older patients; the ability of caspofungin to penetrate the blood-brain barrier and to treat patients with meningitis and endocarditis is unknown.
Caspofungin has not been studied in pediatric patients with endocarditis, osteomyelitis, and meningitis due to Candida. Caspofungin has also not been studied as initial therapy for invasive aspergillosis in pediatric patients.
In clinical trials, 171 pediatric patients (0 months to 17 years of age), including 18 patients who were less than 3 months of age, were given intravenous caspofungin. Pharmacokinetic studies enrolled a total of 66 pediatric patients, and an additional 105 pediatric patients received caspofungin in safety and efficacy studies
Postmarketing hepatobiliary adverse reactions have been reported in pediatric patients with serious underlying medical conditions
•
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), caspofungin acetate for injection 35 mg once daily is recommended based upon pharmacokinetic data
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), caspofungin 35 mg once daily is recommended based upon pharmacokinetic data
Adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) do not need a dosage adjustment. For adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9), caspofungin 35 mg once daily is recommended based upon pharmacokinetic data
Adult and pediatric pharmacokinetic parameters are presented in Table 8.
Plasma concentrations of caspofungin decline in a polyphasic manner following single 1-hour IV infusions. A short α-phase occurs immediately postinfusion, followed by a β-phase (half-life of 9 to 11 hours) that characterizes much of the profile and exhibits clear log-linear behavior from 6 to 48 hours postdose during which the plasma concentration decreases 10-fold. An additional, longer half-life phase, γ-phase, (half-life of 40 to 50 hours), also occurs. Distribution, rather than excretion or biotransformation, is the dominant mechanism influencing plasma clearance. Caspofungin is extensively bound to albumin (~97%), and distribution into red blood cells is minimal. Mass balance results showed that approximately 92% of the administered radioactivity was distributed to tissues by 36 to 48 hours after a single 70 mg dose of [3H] caspofungin acetate. There is little excretion or biotransformation of caspofungin during the first 30 hours after administration.
Caspofungin is slowly metabolized by hydrolysis and N-acetylation. Caspofungin also undergoes spontaneous chemical degradation to an open-ring peptide compound, L-747969. At later time points (≥5 days postdose), there is a low level (≤7 picomoles/mg protein, or ≤1.3% of administered dose) of covalent binding of radiolabel in plasma following single-dose administration of [3H] caspofungin acetate, which may be due to two reactive intermediates formed during the chemical degradation of caspofungin to L-747969. Additional metabolism involves hydrolysis into constitutive amino acids and their degradates, including dihydroxyhomotyrosine and N-acetyl-dihydroxyhomotyrosine. These two tyrosine derivatives are found only in urine, suggesting rapid clearance of these derivatives by the kidneys.
Two single-dose radiolabeled pharmacokinetic studies were conducted. In one study, plasma, urine, and feces were collected over 27 days, and in the second study plasma was collected over 6 months. Plasma concentrations of radioactivity and of caspofungin were similar during the first 24 to 48 hours postdose; thereafter drug levels fell more rapidly. In plasma, caspofungin concentrations fell below the limit of quantitation after 6 to 8 days postdose, while radiolabel fell below the limit of quantitation at 22.3 weeks postdose. After single intravenous administration of [3H] caspofungin acetate, excretion of caspofungin and its metabolites in humans was 35% of dose in feces and 41% of dose in urine. A small amount of caspofungin is excreted unchanged in urine (~1.4% of dose). Renal clearance of parent drug is low (~0.15 mL/min) and total clearance of caspofungin is 12 mL/min.
In a clinical study of single 70 mg doses, caspofungin pharmacokinetics were similar in healthy adult volunteers with mild renal impairment (creatinine clearance 50 to 80 mL/min) and control subjects. Moderate (creatinine clearance 31 to 49 mL/min), severe (creatinine clearance 5 to 30 mL/min), and end-stage (creatinine clearance less than 10 mL/min and dialysis dependent) renal impairment moderately increased caspofungin plasma concentrations after single-dose administration (range: 30 to 49% for AUC). However, in adult patients with invasive aspergillosis, candidemia, or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections) who received multiple daily doses of caspofungin 50 mg, there was no significant effect of mild to end-stage renal impairment on caspofungin concentrations. No dosage adjustment is necessary for patients with renal impairment. Caspofungin is not dialyzable, thus supplementary dosing is not required following hemodialysis.
Plasma concentrations of caspofungin after a single 70 mg dose in adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) were increased by approximately 55% in AUC compared to healthy control subjects. In a 14-day multiple-dose study (70 mg on Day 1 followed by 50 mg daily thereafter), plasma concentrations in adult patients with mild hepatic impairment were increased modestly (19 to 25% in AUC) on Days 7 and 14 relative to healthy control subjects. No dosage adjustment is recommended for patients with mild hepatic impairment.
Adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9) who received a single 70 mg dose of caspofungin had an average plasma caspofungin increase of 76% in AUC compared to control subjects. A dosage reduction is recommended for adult patients with moderate hepatic impairment based upon these pharmacokinetic data
There is no clinical experience in adult patients with severe hepatic impairment (Child-Pugh score greater than 9) or in pediatric patients with any degree of hepatic impairment.
Plasma concentrations of caspofungin in healthy adult men and women were similar following a single 70 mg dose. After 13 daily 50 mg doses, caspofungin plasma concentrations in women were elevated slightly (approximately 22% in area under the curve [AUC]) relative to men. No dosage adjustment is necessary based on gender.
Regression analyses of patient pharmacokinetic data indicated that no clinically significant differences in the pharmacokinetics of caspofungin were seen among Caucasians, Blacks, and Hispanics. No dosage adjustment is necessary on the basis of race.
Plasma concentrations of caspofungin in healthy older men and women (65 years of age and older) were increased slightly (approximately 28% AUC) compared to young healthy men after a single 70 mg dose of caspofungin. In patients who were treated empirically or who had candidemia or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections), a similar modest effect of age was seen in older patients relative to younger patients. No dosage adjustment is necessary for the elderly
Caspofungin has been studied in five prospective studies involving pediatric patients under 18 years of age, including three pediatric pharmacokinetic studies [initial study in adolescents (12 - 17 years of age) and children (2 - 11 years of age) followed by a study in younger patients (3 - 23 months of age) and then followed by a study in neonates and infants (less than 3 months of age)]
Pharmacokinetic parameters following multiple doses of caspofungin in pediatric and adult patients are presented in Table 8.
Population | N | Daily Dose | Pharmacokinetic Parameters (Mean ± Standard Deviation) | |||||
AUC0-24hr ( mcg∙hr/mL) | C1hr ( mcg/mL) | C24hr ( mcg/mL) | t1/2 (hr)* | CI (mL/min) | ||||
PEDIATRIC PATIENTS | ||||||||
| Adolescents, Aged 12-17 years | 8 | 50 mg/m2 | 124.9 ± 50.4 | 14.0 ± 6.9 | 2.4 ± 1.0 | 11.2 ± 1.7 | 12.6 ± 5.5 | |
| Children, Aged 2-11 years | 9 | 50 mg/m2 | 120.0 ± 33.4 | 16.1 ± 4.2 | 1.7 ± 0.8 | 8.2 ± 2.4 | 6.4 ± 2.6 | |
| Young Children, Aged 3-23 months | 8 | 50 mg/m2 | 131.2 ± 17.7 | 17.6 ± 3.9 | 1.7 ± 0.7 | 8.8 ± 2.1 | 3.2 ± 0.4 | |
ADULT PATIENTS | ||||||||
| Adults with Esophageal Candidiasis | 6 † | 50 mg | 87.3 ± 30.0 | 8.7 ± 2.1 | 1.7 ± 0.7 | 13.0 ± 1.9 | 10.6 ± 3.8 | |
| Adults receiving Empirical Therapy | 119 ‡ | 50 mg § | -- | 8.0 ± 3.4 | 1.6 ± 0.7 | -- | -- | |
* Harmonic Mean ± jackknife standard deviation† N=5 for C1hrand AUC0-24hr; N=6 for C24hr‡ N=117 for C24hr; N=119 for C1hr§ Following an initial 70 mg loading dose on day 1 | ||||||||
In clinical studies, caspofungin did not induce the CYP3A4 metabolism of other drugs. Clinical studies in adult healthy volunteers also demonstrated that the pharmacokinetics of caspofungin are not altered by itraconazole, amphotericin B, mycophenolate, nelfinavir, or tacrolimus. Caspofungin has no effect on the pharmacokinetics of itraconazole, amphotericin B, or the active metabolite of mycophenolate.
Adults: Results from regression analyses of adult patient pharmacokinetic data suggest that co-administration of other hepatic CYP enzyme inducers (e.g., efavirenz, nevirapine, phenytoin, dexamethasone, or carbamazepine) with caspofungin may result in clinically meaningful reductions in caspofungin concentrations. It is not known which drug clearance mechanism involved in caspofungin disposition may be inducible
Adult and pediatric pharmacokinetic parameters are presented in Table 8.
Plasma concentrations of caspofungin decline in a polyphasic manner following single 1-hour IV infusions. A short α-phase occurs immediately postinfusion, followed by a β-phase (half-life of 9 to 11 hours) that characterizes much of the profile and exhibits clear log-linear behavior from 6 to 48 hours postdose during which the plasma concentration decreases 10-fold. An additional, longer half-life phase, γ-phase, (half-life of 40 to 50 hours), also occurs. Distribution, rather than excretion or biotransformation, is the dominant mechanism influencing plasma clearance. Caspofungin is extensively bound to albumin (~97%), and distribution into red blood cells is minimal. Mass balance results showed that approximately 92% of the administered radioactivity was distributed to tissues by 36 to 48 hours after a single 70 mg dose of [3H] caspofungin acetate. There is little excretion or biotransformation of caspofungin during the first 30 hours after administration.
Caspofungin is slowly metabolized by hydrolysis and N-acetylation. Caspofungin also undergoes spontaneous chemical degradation to an open-ring peptide compound, L-747969. At later time points (≥5 days postdose), there is a low level (≤7 picomoles/mg protein, or ≤1.3% of administered dose) of covalent binding of radiolabel in plasma following single-dose administration of [3H] caspofungin acetate, which may be due to two reactive intermediates formed during the chemical degradation of caspofungin to L-747969. Additional metabolism involves hydrolysis into constitutive amino acids and their degradates, including dihydroxyhomotyrosine and N-acetyl-dihydroxyhomotyrosine. These two tyrosine derivatives are found only in urine, suggesting rapid clearance of these derivatives by the kidneys.
Two single-dose radiolabeled pharmacokinetic studies were conducted. In one study, plasma, urine, and feces were collected over 27 days, and in the second study plasma was collected over 6 months. Plasma concentrations of radioactivity and of caspofungin were similar during the first 24 to 48 hours postdose; thereafter drug levels fell more rapidly. In plasma, caspofungin concentrations fell below the limit of quantitation after 6 to 8 days postdose, while radiolabel fell below the limit of quantitation at 22.3 weeks postdose. After single intravenous administration of [3H] caspofungin acetate, excretion of caspofungin and its metabolites in humans was 35% of dose in feces and 41% of dose in urine. A small amount of caspofungin is excreted unchanged in urine (~1.4% of dose). Renal clearance of parent drug is low (~0.15 mL/min) and total clearance of caspofungin is 12 mL/min.
In a clinical study of single 70 mg doses, caspofungin pharmacokinetics were similar in healthy adult volunteers with mild renal impairment (creatinine clearance 50 to 80 mL/min) and control subjects. Moderate (creatinine clearance 31 to 49 mL/min), severe (creatinine clearance 5 to 30 mL/min), and end-stage (creatinine clearance less than 10 mL/min and dialysis dependent) renal impairment moderately increased caspofungin plasma concentrations after single-dose administration (range: 30 to 49% for AUC). However, in adult patients with invasive aspergillosis, candidemia, or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections) who received multiple daily doses of caspofungin 50 mg, there was no significant effect of mild to end-stage renal impairment on caspofungin concentrations. No dosage adjustment is necessary for patients with renal impairment. Caspofungin is not dialyzable, thus supplementary dosing is not required following hemodialysis.
Plasma concentrations of caspofungin after a single 70 mg dose in adult patients with mild hepatic impairment (Child-Pugh score 5 to 6) were increased by approximately 55% in AUC compared to healthy control subjects. In a 14-day multiple-dose study (70 mg on Day 1 followed by 50 mg daily thereafter), plasma concentrations in adult patients with mild hepatic impairment were increased modestly (19 to 25% in AUC) on Days 7 and 14 relative to healthy control subjects. No dosage adjustment is recommended for patients with mild hepatic impairment.
Adult patients with moderate hepatic impairment (Child-Pugh score 7 to 9) who received a single 70 mg dose of caspofungin had an average plasma caspofungin increase of 76% in AUC compared to control subjects. A dosage reduction is recommended for adult patients with moderate hepatic impairment based upon these pharmacokinetic data
There is no clinical experience in adult patients with severe hepatic impairment (Child-Pugh score greater than 9) or in pediatric patients with any degree of hepatic impairment.
Plasma concentrations of caspofungin in healthy adult men and women were similar following a single 70 mg dose. After 13 daily 50 mg doses, caspofungin plasma concentrations in women were elevated slightly (approximately 22% in area under the curve [AUC]) relative to men. No dosage adjustment is necessary based on gender.
Regression analyses of patient pharmacokinetic data indicated that no clinically significant differences in the pharmacokinetics of caspofungin were seen among Caucasians, Blacks, and Hispanics. No dosage adjustment is necessary on the basis of race.
Plasma concentrations of caspofungin in healthy older men and women (65 years of age and older) were increased slightly (approximately 28% AUC) compared to young healthy men after a single 70 mg dose of caspofungin. In patients who were treated empirically or who had candidemia or other Candida infections (intra-abdominal abscesses, peritonitis, or pleural space infections), a similar modest effect of age was seen in older patients relative to younger patients. No dosage adjustment is necessary for the elderly
Caspofungin has been studied in five prospective studies involving pediatric patients under 18 years of age, including three pediatric pharmacokinetic studies [initial study in adolescents (12 - 17 years of age) and children (2 - 11 years of age) followed by a study in younger patients (3 - 23 months of age) and then followed by a study in neonates and infants (less than 3 months of age)]
Pharmacokinetic parameters following multiple doses of caspofungin in pediatric and adult patients are presented in Table 8.
Population | N | Daily Dose | Pharmacokinetic Parameters (Mean ± Standard Deviation) | |||||
AUC0-24hr ( mcg∙hr/mL) | C1hr ( mcg/mL) | C24hr ( mcg/mL) | t1/2 (hr)* | CI (mL/min) | ||||
PEDIATRIC PATIENTS | ||||||||
| Adolescents, Aged 12-17 years | 8 | 50 mg/m2 | 124.9 ± 50.4 | 14.0 ± 6.9 | 2.4 ± 1.0 | 11.2 ± 1.7 | 12.6 ± 5.5 | |
| Children, Aged 2-11 years | 9 | 50 mg/m2 | 120.0 ± 33.4 | 16.1 ± 4.2 | 1.7 ± 0.8 | 8.2 ± 2.4 | 6.4 ± 2.6 | |
| Young Children, Aged 3-23 months | 8 | 50 mg/m2 | 131.2 ± 17.7 | 17.6 ± 3.9 | 1.7 ± 0.7 | 8.8 ± 2.1 | 3.2 ± 0.4 | |
ADULT PATIENTS | ||||||||
| Adults with Esophageal Candidiasis | 6 † | 50 mg | 87.3 ± 30.0 | 8.7 ± 2.1 | 1.7 ± 0.7 | 13.0 ± 1.9 | 10.6 ± 3.8 | |
| Adults receiving Empirical Therapy | 119 ‡ | 50 mg § | -- | 8.0 ± 3.4 | 1.6 ± 0.7 | -- | -- | |
* Harmonic Mean ± jackknife standard deviation† N=5 for C1hrand AUC0-24hr; N=6 for C24hr‡ N=117 for C24hr; N=119 for C1hr§ Following an initial 70 mg loading dose on day 1 | ||||||||
In clinical studies, caspofungin did not induce the CYP3A4 metabolism of other drugs. Clinical studies in adult healthy volunteers also demonstrated that the pharmacokinetics of caspofungin are not altered by itraconazole, amphotericin B, mycophenolate, nelfinavir, or tacrolimus. Caspofungin has no effect on the pharmacokinetics of itraconazole, amphotericin B, or the active metabolite of mycophenolate.
Adults: Results from regression analyses of adult patient pharmacokinetic data suggest that co-administration of other hepatic CYP enzyme inducers (e.g., efavirenz, nevirapine, phenytoin, dexamethasone, or carbamazepine) with caspofungin may result in clinically meaningful reductions in caspofungin concentrations. It is not known which drug clearance mechanism involved in caspofungin disposition may be inducible
Caspofungin is contraindicated in patients with known hypersensitivity (e.g., anaphylaxis) to any component of this product
The following serious adverse reactions are discussed in detail in another section of the labeling:
• Hypersensitivity
• Hepatic Effects
• Elevated Liver Enzymes During Concomitant Use with Cyclosporine
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of caspofungin cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions | Caspofungin* N=564 (%) | AmBisome† N=547 (%) |
All Systems, Any Adverse Reaction | 95 | 97 |
Investigations | 58 | 63 |
| Alanine Aminotransferase Increased | 18 | 20 |
| Blood Alkaline Phosphatase Increased | 15 | 23 |
| Blood Potassium Decreased | 15 | 23 |
| Aspartate Aminotransferase Increased | 14 | 17 |
| Blood Bilirubin Increased | 10 | 14 |
| Blood Magnesium Decreased | 7 | 9 |
| Blood Glucose Increased | 6 | 9 |
| Bilirubin Conjugated Increased | 5 | 9 |
| Blood Urea Increased | 4 | 8 |
| Blood Creatinine Increased | 3 | 11 |
General Disorders and Administration Site Conditions | 57 | 63 |
| Pyrexia | 27 | 29 |
| Chills | 23 | 31 |
| Edema Peripheral | 11 | 12 |
| Mucosal Inflammation | 6 | 8 |
Gastrointestinal Disorders | 50 | 55 |
| Diarrhea | 20 | 16 |
| Nausea | 11 | 20 |
| Abdominal Pain | 9 | 11 |
| Vomiting | 9 | 17 |
Respiratory, Thoracic and Mediastinal Disorders | 47 | 49 |
| Dyspnea | 9 | 10 |
Skin and Subcutaneous Tissue Disorders | 42 | 37 |
| Rash | 16 | 14 |
Nervous System Disorders | 25 | 27 |
| Headache | 11 | 12 |
Metabolism and Nutrition Disorders | 21 | 24 |
| Hypokalemia | 6 | 8 |
Vascular Disorders | 20 | 23 |
| Hypotension | 6 | 10 |
Cardiac Disorders | 16 | 19 |
| Tachycardia | 7 | 9 |
| Within any system organ class, individuals may experience more than 1 adverse reaction. * 70 mg on Day 1, then 50 mg once daily for the remainder of treatment; daily dose was increased to 70 mg for 73 patients.† 3 mg/kg/day; daily dose was increased to 5 mg/kg for 74 patients. | ||
The proportion of patients who experienced an infusion-related adverse reaction (defined as a systemic event, such as pyrexia, chills, flushing, hypotension, hypertension, tachycardia, dyspnea, tachypnea, rash, or anaphylaxis, that developed during the study therapy infusion and one hour following infusion) was significantly lower in the group treated with caspofungin (35%) than in the group treated with AmBisome (52%).
To evaluate the effect of caspofungin and AmBisome on renal function, nephrotoxicity was defined as doubling of serum creatinine relative to baseline or an increase of greater than or equal to 1 mg/dL in serum creatinine if baseline serum creatinine was above the upper limit of the normal range. Among patients whose baseline creatinine clearance was greater than 30 mL/min, the incidence of nephrotoxicity was significantly lower in the group treated with caspofungin (3%) than in the group treated with AmBisome (12%).
In the randomized, double-blinded invasive candidiasis study, patients received either caspofungin 50 mg/day (following a 70 mg loading dose) or amphotericin B 0.6 to 1 mg/kg/day. Adverse reactions occurring in 10% or greater of the patients in either treatment group are presented in Table 3.
Adverse Reactions | Caspofungin 50 mg† N=114 (%) | Amphotericin B N=125 (%) |
All Systems, Any Adverse Reaction | 96 | 99 |
Investigations | 67 | 82 |
| Blood Potassium Decreased | 23 | 32 |
| Blood Alkaline Phosphatase Increased | 21 | 32 |
| Hemoglobin Decreased | 18 | 23 |
| Alanine Aminotransferase Increased | 16 | 15 |
| Aspartate Aminotransferase Increased | 16 | 14 |
| Blood Bilirubin Increased | 13 | 17 |
| Hematocrit Decreased | 13 | 18 |
| Blood Creatinine Increased | 11 | 28 |
| Red Blood Cells Urine Positive | 10 | 10 |
| Blood Urea Increased | 9 | 23 |
| Bilirubin Conjugated Increased | 8 | 14 |
Gastrointestinal Disorders | 49 | 53 |
| Vomiting | 17 | 16 |
| Diarrhea | 14 | 10 |
| Nausea | 9 | 17 |
General Disorders and Administration Site Conditions | 47 | 63 |
| Pyrexia | 13 | 33 |
| Edema Peripheral | 11 | 12 |
| Chills | 9 | 30 |
Respiratory, Thoracic and Mediastinal Disorders | 40 | 54 |
| Tachypnea | 1 | 11 |
Cardiac Disorders | 26 | 34 |
| Tachycardia | 8 | 12 |
Skin and Subcutaneous Tissue Disorders | 25 | 28 |
| Rash | 4 | 10 |
Vascular Disorders | 25 | 38 |
| Hypotension | 10 | 16 |
Blood and Lymphatic System Disorders | 15 | 13 |
| Anemia | 11 | 9 |
| Within any system organ class, individuals may experience more than 1 adverse reaction. * Intra-abdominal abscesses, peritonitis and pleural space infections.† Patients received caspofungin 70 mg on Day 1, then 50 mg once daily for the remainder of their treatment. | ||
The proportion of patients who experienced an infusion-related adverse reaction (defined as a systemic event, such as pyrexia, chills, flushing, hypotension, hypertension, tachycardia, dyspnea, tachypnea, rash, or anaphylaxis, that developed during the study therapy infusion and one hour following infusion) was significantly lower in the group treated with caspofungin (20%) than in the group treated with amphotericin B (49%).
To evaluate the effect of caspofungin and amphotericin B on renal function, nephrotoxicity was defined as doubling of serum creatinine relative to baseline or an increase of greater than or equal to 1 mg/dL in serum creatinine if baseline serum creatinine was above the upper limit of the normal range. In a subgroup of patients whose baseline creatinine clearance was greater than 30 mL/min, the incidence of nephrotoxicity was significantly lower in the group treated with caspofungin than in the group treated with amphotericin B.
In a second randomized, double-blinded invasive candidiasis study, patients received either caspofungin 50 mg/day (following a 70 mg loading dose) or caspofungin 150 mg/day. The proportion of patients who experienced any adverse reaction was similar in the 2 treatment groups; however, this study was not large enough to detect differences in rare or unexpected adverse reactions. Adverse reactions occurring in 5% or greater of the patients in either treatment group are presented in Table 4.
Adverse Reactions | Caspofungin 50 mg† N=104 (%) | Caspofungin 150 mg N=100 (%) |
All Systems, Any Adverse Reaction | 83 | 83 |
General Disorders and Administration Site Conditions | 33 | 27 |
| Pyrexia | 6 | 6 |
Gastrointestinal Disorders | 30 | 33 |
| Vomiting | 11 | 6 |
| Diarrhea | 6 | 7 |
| Nausea | 5 | 7 |
Investigations | 28 | 35 |
| Alkaline Phosphatase Increased | 12 | 9 |
| Aspartate Aminotransferase Increased | 6 | 9 |
| Blood Potassium Decreased | 6 | 8 |
| Alanine Aminotransferase Increased | 4 | 7 |
Vascular Disorders | 19 | 18 |
| Hypotension | 7 | 3 |
| Hypertension | 5 | 6 |
| Within any system organ class, individuals may experience more than 1 adverse event. * Intra-abdominal abscesses, peritonitis and pleural space infections.† Patients received caspofungin 70 mg on Day 1, then 50 mg once daily for the remainder of their treatment. | ||
Adverse reactions occurring in 10% or greater of patients with esophageal and/or oropharyngeal candidiasis are presented in Table 5.
Adverse Reactions | Caspofungin 50 mg* N=83 (%) | Fluconazole IV 200 mg* N=94 (%) |
All Systems, Any Adverse Reaction | 90 | 93 |
Gastrointestinal Disorders | 58 | 50 |
| Diarrhea | 27 | 18 |
| Nausea | 15 | 15 |
Investigations | 53 | 61 |
| Hemoglobin Decreased | 21 | 16 |
| Hematocrit Decreased | 18 | 16 |
| Aspartate Aminotransferase Increased | 13 | 19 |
| Blood Alkaline Phosphatase Increased | 13 | 17 |
| Alanine Aminotransferase Increased | 12 | 17 |
| White Blood Cell Count Decreased | 12 | 19 |
General Disorders and Administration Site Conditions | 31 | 36 |
| Pyrexia | 21 | 21 |
Vascular Disorders | 19 | 15 |
| Phlebitis | 18 | 11 |
Nervous System Disorders | 18 | 17 |
| Headache | 15 | 9 |
| Within any system organ class, individuals may experience more than 1 adverse reaction. * Derived from a comparator-controlled clinical study. | ||
In an open-label, noncomparative aspergillosis study, in which 69 patients received caspofungin
(70 mg loading dose on Day 1 followed by 50 mg daily), the following adverse reactions were observed with an incidence of 12.5% or greater: blood alkaline phosphatase increased (22%), hypotension (20%), respiratory failure (20%), pyrexia (17%), diarrhea (15%), nausea (15%), headache (15%), rash (13%), alanine aminotransferase increased (13%), aspartate aminotransferase increased (13%), blood bilirubin increased (13%), and blood potassium decreased (13%). Also reported in this patient population were pulmonary edema, ARDS (adult respiratory distress syndrome), and radiographic infiltrates.
The overall safety of caspofungin was assessed in 171 pediatric patients who received single or multiple doses of caspofungin. The distribution among the 153 pediatric patients who were over the age of 3 months was as follows: 104 febrile, neutropenic patients; 38 patients with candidemia and/or intra-abdominal abscesses, peritonitis, or pleural space infections; 1 patient with esophageal candidiasis; and 10 patients with invasive aspergillosis. The overall safety profile of caspofungin in pediatric patients is comparable to that in adult patients. Table 6 shows the incidence of adverse reactions reported in 7.5% or greater of pediatric patients in clinical studies.
One patient (0.6%) receiving caspofungin, and three patients (12%) receiving AmBisome developed a serious drug-related adverse reaction. Two patients (1%) were discontinued from caspofungin and three patients (12%) were discontinued from AmBisome due to a drug-related adverse reaction. The proportion of patients who experienced an infusion-related adverse reaction (defined as a systemic event, such as pyrexia, chills, flushing, hypotension, hypertension, tachycardia, dyspnea, tachypnea, rash, or anaphylaxis, that developed during the study therapy infusion and one hour following infusion) was 22% in the group treated with caspofungin and 35% in the group treated with AmBisome.
Adverse Reactions | Noncomparative Clinical Studies | Comparator-Controlled Clinical Study of Empirical Therapy | |
Caspofungin Any Dose N=115 (%) | Caspofungin 50 mg/m2* N=56 (%) | AmBisome 3 mg/kg N=26 (%) | |
All Systems, Any Adverse Reaction | 95 | 96 | 89 |
Investigations | 55 | 41 | 50 |
| Blood Potassium Decreased | 18 | 9 | 27 |
| Aspartate Aminotransferase Increased | 17 | 2 | 12 |
| Alanine Aminotransferase Increased | 14 | 5 | 12 |
| Blood Potassium Increased | 3 | 0 | 8 |
General Disorders and Administration Site Conditions | 47 | 59 | 42 |
| Pyrexia | 29 | 30 | 23 |
| Chills | 10 | 13 | 8 |
| Mucosal Inflammation | 10 | 4 | 4 |
| Edema | 3 | 4 | 8 |
Gastrointestinal Disorders | 42 | 41 | 35 |
| Diarrhea | 17 | 7 | 15 |
| Vomiting | 8 | 11 | 12 |
| Abdominal Pain | 7 | 4 | 12 |
| Nausea | 4 | 4 | 8 |
Infections and Infestations | 40 | 30 | 35 |
| Central Line Infection | 1 | 9 | 0 |
Skin and Subcutaneous Tissue Disorders | 33 | 41 | 39 |
| Pruritus | 7 | 6 | 8 |
| Rash | 6 | 23 | 8 |
| Erythema | 4 | 9 | 0 |
Vascular Disorders | 24 | 21 | 19 |
| Hypotension | 12 | 9 | 8 |
| Hypertension | 10 | 9 | 4 |
Metabolism and Nutrition Disorders | 22 | 11 | 23 |
| Hypokalemia | 8 | 5 | 4 |
Cardiac Disorders | 17 | 13 | 19 |
| Tachycardia | 4 | 11 | 19 |
Nervous System Disorders | 13 | 16 | 8 |
| Headache | 5 | 9 | 4 |
Musculoskeletal and Connective Tissue Disorders | 11 | 14 | 12 |
| Back Pain | 4 | 0 | 8 |
Blood and Lymphatic System Disorders | 10 | 2 | 15 |
| Anemia | 2 | 0 | 8 |
| Within any system organ class, individuals may experience more than 1 adverse reaction. * 70 mg/m2on Day 1, then 50 mg/m2once daily for the remainder of the treatment. | |||
The overall safety of caspofungin was assessed in 2036 individuals (including 1642 adult or pediatric patients and 394 volunteers) from 34 clinical studies. These individuals received single or multiple (once daily) doses of caspofungin, ranging from 5 mg to 210 mg. Full safety data is available from 1951 individuals, as the safety data from 85 patients enrolled in 2 compassionate use studies was limited solely to serious adverse reactions. Adverse reactions which occurred in 5% or greater of all individuals who received caspofungin in these trials are shown in Table 7.
Overall, 1665 of the 1951 (85%) patients/volunteers who received caspofungin experienced an adverse reaction.
Adverse Reactions‡ | Caspofungin (N=1951) | |
n | (%) | |
All Systems, Any Adverse Reaction | 1665 | (85) |
Investigations | 901 | (46) |
| Alanine Aminotransferase Increased | 258 | (13) |
| Aspartate Aminotransferase Increased | 233 | (12) |
| Blood Alkaline Phosphatase Increased | 232 | (12) |
| Blood Potassium Decreased | 220 | (11) |
| Blood Bilirubin Increased | 117 | (6) |
General Disorders and Administration Site Conditions | 843 | (43) |
| Pyrexia | 381 | (20) |
| Chills | 192 | (10) |
| Edema Peripheral | 110 | (6) |
Gastrointestinal Disorders | 754 | (39) |
| Diarrhea | 273 | (14) |
| Nausea | 166 | (9) |
| Vomiting | 146 | (8) |
| Abdominal Pain | 112 | (6) |
Infections and Infestations | 730 | (37) |
| Pneumonia | 115 | (6) |
Respiratory, Thoracic, and Mediastinal Disorders | 613 | (31) |
| Cough | 111 | (6) |
Skin and Subcutaneous Tissue Disorders | 520 | (27) |
| Rash | 159 | (8) |
| Erythema | 98 | (5) |
Nervous System Disorders | 412 | (21) |
| Headache | 193 | (10) |
Vascular Disorders | 344 | (18) |
| Hypotension | 118 | (6) |
* Defined as an adverse reaction, regardless of causality, while on caspofungin or during the 14-day post- caspofungin follow-up period.† Incidence for each preferred term is 5% or greater among individuals who received at least 1 dose of caspofungin.‡ Within any system organ class, individuals may experience more than 1 adverse event. | ||
Clinically significant adverse reactions, regardless of causality or incidence which occurred in less than 5% of patients are listed below.
The following additional adverse reactions have been identified during the post-approval use of caspofungin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
•
Anaphylaxis and other hypersensitivity reactions have been reported during administration of caspofungin.
Possible histamine-mediated adverse reactions, including rash, facial swelling, angioedema, pruritus, sensation of warmth or bronchospasm have been reported.
Cases of Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), some with a fatal outcome, have been reported with use of caspofungin
Discontinue caspofungin at the first sign or symptom of a hypersensitivity reaction and administer appropriate treatment.
•
Laboratory abnormalities in liver function tests have been seen in healthy volunteers and in adult and pediatric patients treated with caspofungin. In some adult and pediatric patients with serious underlying conditions who were receiving multiple concomitant medications with caspofungin, isolated cases of clinically significant hepatic dysfunction, hepatitis, and hepatic failure have been reported; a causal relationship to caspofungin has not been established. Monitor patients who develop abnormal liver function tests during caspofungin therapy for evidence of worsening hepatic function and evaluated for risk/benefit of continuing caspofungin therapy.
•
Elevated liver enzymes have occurred in patients receiving caspofungin and cyclosporine concomitantly. Only use caspofungin and cyclosporine in those patients for whom the potential benefit outweighs the potential risk. Patients who develop abnormal liver enzymes during concomitant therapy should be monitored and the risk/benefit of continuing therapy should be evaluated.