Caverject Impulse
(Alprostadil)Caverject Impulse Prescribing Information
CAVERJECT IMPULSE is a prostaglandin E1 agonist indicated
• For the treatment of erectile dysfunction ()1.1 Erectile DysfunctionCAVERJECT IMPULSE is indicated for the treatment of erectile dysfunction.
• As an adjunct to other diagnostic tests in the diagnosis of erectile dysfunction ().1.2 Diagnostic TestCAVERJECT IMPULSE is indicated as an adjunct to other diagnostic tests in the diagnosis of erectile dysfunction.
• Determine the most suitable dose and presentation of CAVERJECT to use. Use a new syringe for each dose of CAVERJECT ().2.1 Important Dosage and Administration Instructions• Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.• Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.• CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.• Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.• Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
• Administer first intracavernosal injections in the health care provider’s office and titrate the dose for each patient to the lowest effective dose ().2.1 Important Dosage and Administration Instructions• Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.• Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.• CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.• Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.• Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of at-home use ().2.1 Important Dosage and Administration Instructions• Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.• Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.• CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.• Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.• Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
• Recommended dosage for erectile dysfunction ():2.2 Recommended Dosage for Erectile DysfunctionErectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology.• Initiate dosing with 2.5 mcg of CAVERJECT IMPULSE intracavernousally as recommended[see Dosage and Administration 2.4].• If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour.• During titration, no more than 2 doses should be given within a 24-hour period.• If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury):• Initiate dosing with 1.25 mcg of alprostadil using CAVERJECT.• If there is a partial response, administer another dose of CAVERJECT of 1.25 mcg within 1 hour.• No more than 2 doses during initial titration should be given within a 24-hour period.• If additional titration is required, administer a dose of 5 mcg at least 24 hours later.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use:• Once the dose of CAVERJECT IMPULSE has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.• The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile DysfunctionTo diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging. For any of these tests, use a single dose of CAVERJECT IMPULSE that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
o Erectile dysfunction of vasculogenic, psychogenic, or mixed etiology:
Initiate dosing with 2.5 mcgo Erectile dysfunction of pure neurogenic etiology (spinal cord injury):
Initiate dosing with 1.25 mcg
• Follow dose titration procedures for each type of erectile dysfunction and determine the maintenance dosage for patient home use in the health care provider’s office ().2.2 Recommended Dosage for Erectile DysfunctionErectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology.• Initiate dosing with 2.5 mcg of CAVERJECT IMPULSE intracavernousally as recommended[see Dosage and Administration 2.4].• If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour.• During titration, no more than 2 doses should be given within a 24-hour period.• If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury):• Initiate dosing with 1.25 mcg of alprostadil using CAVERJECT.• If there is a partial response, administer another dose of CAVERJECT of 1.25 mcg within 1 hour.• No more than 2 doses during initial titration should be given within a 24-hour period.• If additional titration is required, administer a dose of 5 mcg at least 24 hours later.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use:• Once the dose of CAVERJECT IMPULSE has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.• The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile DysfunctionTo diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging. For any of these tests, use a single dose of CAVERJECT IMPULSE that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
• The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose ().2.2 Recommended Dosage for Erectile DysfunctionErectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology.• Initiate dosing with 2.5 mcg of CAVERJECT IMPULSE intracavernousally as recommended[see Dosage and Administration 2.4].• If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour.• During titration, no more than 2 doses should be given within a 24-hour period.• If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury):• Initiate dosing with 1.25 mcg of alprostadil using CAVERJECT.• If there is a partial response, administer another dose of CAVERJECT of 1.25 mcg within 1 hour.• No more than 2 doses during initial titration should be given within a 24-hour period.• If additional titration is required, administer a dose of 5 mcg at least 24 hours later.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use:• Once the dose of CAVERJECT IMPULSE has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.• The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile DysfunctionTo diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging. For any of these tests, use a single dose of CAVERJECT IMPULSE that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
• While on self-injection treatment, the patient should visit the prescribing health care provider’s office every 3 months to assess the efficacy and safety of the therapy ().2.1 Important Dosage and Administration Instructions• Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.• Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.• CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.• Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.• Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
• Follow procedure for CAVERJECT IMPULSE syringe preparation ().2.3 Syringe Preparation Instructions1.000000000000000e+00 Select the CAVERJECT IMPULSE syringe based upon dose to be administered.
Syringe StrengthReconstituted ConcentrationDosages Available for Delivery after Reconstitution10 mcg
10 mcg/0.5 mL
2.5 mcg
5 mcg
7.5 mcg
10 mcg
20 mcg
20 mcg/0.5 mL
5 mcg
10 mcg
15 mcg
20 mcg
2.000000000000000e+00 Open the sealed plastic tray. Remove the syringe, the needle assembly, and the alcohol swabs from the tray. The syringe has a dose window and a plunger. The needle assembly is a sealed unit that contains the outer protective cap, the inner protective cap, and the superfine needle.3.000000000000000e+00 Use the alcohol swab to wipe the rubber membrane at the tip of the syringe. Pick up the needle assembly, grasp the paper tab, and peel off the paper cover (the lid).4.000000000000000e+00 Hold the needle assembly by the cap and press the needle assembly onto the tip of the syringe. Turn it clockwise until the needle assembly is firmly locked into place.5.000000000000000e+00 Remove the outer protective cap from the needle by twisting it clockwise. Do not yet remove the inner protective cap, the thin plastic tube that directly covers the needle.6.000000000000000e+00 Hold the syringe system with the needle pointing upward. The plunger rod should still be in the fully extended position, with all of the threads visible. Slowly rotate the plunger rod clockwise until it goes all the way in and stops. Do not push on the plunger while trying to rotate it.7.000000000000000e+00 Turn the syringe upside down several times to make sure the solution is evenly mixed. The solution should be clear. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. The product should not be used if particulate matter or discoloration are present.8.000000000000000e+00 Hold the syringe with the needle upward and carefully remove the inner protective cap from the needle. Lightly tap the glass cartridge a few times with your finger until any large bubbles disappear up into the tip. With the syringe pointed upward, push in the plunger rod until it stops to push any air out.9.000000000000000e+00 To set the dose: locate the dose window on the syringe and then slowly turn the plunger rod clockwise until the correct dose number appears in the center of the window. The syringe is now ready for use. If you pass the correct number, keep turning the plunger in the same direction until the correct number comes around again – do not try to turn it backward.1.000000000000000e+01 After reconstitution, the syringe should be used within 24 hours when stored between 36 to 77°F (2°C to 25°C). Do not freeze. CAVERJECT IMPULSE is for single use only. Discard the injection delivery system and any remaining solution after use.
• Follow the procedure for CAVERJECT IMPULSE administration ().2.4 Administration Instructions• Administer CAVERJECT IMPUSLE intracavernosally along the dorso-lateral aspect of the proximal third of the penis. See Figures Aand Bbelow.
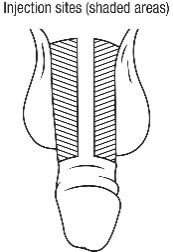 Figure A
Figure A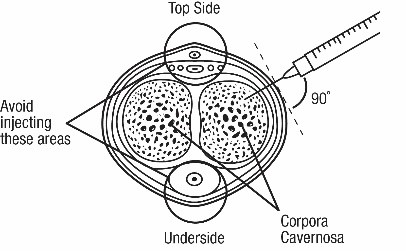 Figure B
Figure B• Wipe the intended injection site with an alcohol swab prior to injection.• Avoid visible veins during injection.• Alternate the side of the penis that is injected and the site of injection.• Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes.• Each CAVERJECT IMPULSE syringe is intended for single use only (one dose only) and should be discarded after use.

Injection site 
Top side • To diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection ().2.2 Recommended Dosage for Erectile DysfunctionErectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology.• Initiate dosing with 2.5 mcg of CAVERJECT IMPULSE intracavernousally as recommended[see Dosage and Administration 2.4].• If there is a partial response at 2.5 mcg, administer another dose of 2.5 mcg within 1 hour.• During titration, no more than 2 doses should be given within a 24-hour period.• If additional titration is required, administer doses in increments of 5 to 10 mcg at least 24 hours apart.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Erectile Dysfunction of Pure Neurogenic Etiology (Spinal Cord Injury):• Initiate dosing with 1.25 mcg of alprostadil using CAVERJECT.• If there is a partial response, administer another dose of CAVERJECT of 1.25 mcg within 1 hour.• No more than 2 doses during initial titration should be given within a 24-hour period.• If additional titration is required, administer a dose of 5 mcg at least 24 hours later.• The optimal dose should produce an erection suitable for intercourse that does not exceed a duration of 1 hour.• The patient must stay in the health care provider’s office until complete detumescence occurs.
Repeat the titration as necessary until the optimal dose is achieved. Doses greater than 60 mcg are not recommended.
Maintenance Dosage for Patient Home Use:• Once the dose of CAVERJECT IMPULSE has been determined in the health care provider’s office, additional dose adjustment may be required after consultation with the health care provider. Adjust the dose in accordance with the titration guidelines described above.• The recommended frequency of injection is no more than 3 times weekly, with at least 24 hours between each dose.
Adjunct to the Diagnosis of Erectile DysfunctionTo diagnose erectile dysfunction (pharmacologic testing), inject CAVERJECT IMPULSE intracavernosally and monitor patients for the occurrence of an erection. Extensions of this testing are the use of CAVERJECT as an adjunct to laboratory investigations, such as duplex or Doppler imaging. For any of these tests, use a single dose of CAVERJECT IMPULSE that induces a rigid erection. Use the dose regimen for ‘Erectile Dysfunction of Vasculogenic, Psychogenic, or Mixed Etiology’ above.
Caverject Impulse (alprostadil) for injection contains sterile, freeze-dried alprostadil for reconstitution and sterile bacteriostatic water in a prefilled dual chamber glass cartridge. It is available in 10 mcg and 20 mcg strengths.
For injection: 10 mcg and 20 mcg of alprostadil freeze-dried powder for reconstitution in single-dose, pre‑filled, dual chambered glass cartridge syringe. The front chamber contains alprostadil and the rear chamber contains sterile bacteriostatic water. A 29 gauge needle system is also included.
The reconstituted solution is clear.
CAVERJECT IMPULSE is not indicated for use in females.
CAVERJECT IMPULSE is contraindicated:
• in men who have a known hypersensitivity to the drug[see]6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
CAVERJECT IMPULSE was evaluated in 87 patients in an open-label crossover study of 6 weeks treatment duration that compared the formulation of alprostadil for injection contained in CAVERJECT IMPULSE with the formulation contained in CAVERJECT. Doses used in this study ranged from 2.5 mcg to 20 mcg. Adverse reactions reported for the CAVERJECT IMPULSE formulation included: penis disorder (4.6%), prolonged erection (1.1%), injection site erythema (1.1%), rash (1.1%), dizziness (1.1%), and hematospermia (1.1%). Penis disorder included penile pain, post-injection pain, and pain with erection.
CAVERJECT IMPULSE was also evaluated in 63 patients in a single-dose, double-blind, crossover study that compared CAVERJECT IMPULSE with CAVERJECT. Doses used in this study ranged from 2.5 mcg to 20 mcg. Adverse reactions reported for the CAVERJECT IMPULSE formulation included: penile pain (1.6%) and pruritus (1.6%).
In addition to the adverse reactions observed for CAVERJECT IMPULSE in these two studies, the following adverse reactions have been reported in clinical studies of CAVERJECT:
Local Adverse Reactions:Local adverse reactions derived from 1861 patients in clinical studies of CAVERJECT, including an 18-month, open-label study, are shown in Table 1.Table 1. Local Adverse Reactions Reported by ≥ 1% of Patients Treated with CAVERJECT for up to 18 Months Penile pain
37%
Prolonged erection
4%
Penile fibrosis
3%
Injection site hematoma
3%
Penis disorderPenis disorder includes: numbness, irritation, sensitivity, pruritus, erythema, skin tear, discoloration, itching.
3%
Injection site ecchymosis
2%
Penile rash
1%
Penile edema
1%
The following local adverse reactions were reported in < 1% of patients: injection site hemorrhage, injection site inflammation, injection site itching, injection site swelling, injection site edema, urethral bleeding, penile warmth, numbness, irritation, sensitivity, pruritus, erythema, painful erection, and abnormal ejaculation.
In these studies, no local adverse reactions were reported in the 294 patients who received placebo, except for penile pain (2%).
Penile Pain:In the majority of the cases, penile pain was rated mild or moderate in intensity. Three percent of patients discontinued treatment because of penile painProlonged Erection/Priapism:Prolonged erection was defined as an erection that lasted for 4 to 6 hours; priapism was defined as an erection that lasted 6 hours or longer. In clinical studies, the frequency of prolonged erection after CAVERJECT was 4%, while the frequency of priapism was 0.4%[see Warnings and Precautions (5.1)].Penile Hematoma/Ecchymosis:In clinical studies, the frequency of penile hematoma and ecchymosis was 3% and 2%, respectively.Systemic Adverse Reactions:Systemic adverse reactions reported by ≥ 1% of subjects in clinical studies of CAVERJECT included: dizziness (1%).The following systemic adverse reactions were reported in < 1% of patients: testicular pain, scrotal edema, hematuria, pelvic pain, hypotension, vasodilation, vasovagal reaction, diaphoresis, rash, and non-application site pruritus. Three patients (0.2%) discontinued due to symptomatic hypotension.
No systemic adverse reactions were reported in the 294 patients who received placebo.
• in men who have conditions that predispose them to priapism, such as sickle cell anemia or sickle cell trait, multiple myeloma, or leukemia[see]5.1 Prolonged Erection and PriapismProlonged erection, defined as erection lasting between 4 to 6 hours in duration, occurred in 4% of 1,861 patients treated up to 18 months in studies of CAVERJECT. The incidence of priapism (erections lasting more than 6 hours in duration) was 0.4%. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
To minimize the chances of prolonged erection or priapism, titrate CAVERJECT IMPULSE to the lowest effective dose
[seeDosage and Administration (2.1].In addition, do not use CAVERJECT IMPULSE in patients who have conditions that predispose them to priapism, such as sickle cell anemia or sickle cell trait, multiple myeloma, or leukemia[see Contraindications (4)].• for the treatment of erectile dysfunction in men with fibrotic conditions of the penis, such as anatomical deformation, angulation, cavernosal fibrosis, or Peyronie's disease[see]5.2 Penile FibrosisThe overall incidence of penile fibrosis reported in clinical studies with CAVERJECT was 3%. In one self-injection clinical study where duration of use was up to 18 months, the incidence of penile fibrosis was 7.8%.
Physical examination of the penis should be performed periodically to detect signs of penile fibrosis. Treatment with CAVERJECT IMPULSE should be discontinued in patients who develop penile angulation or cavernosal fibrosis.
• in men with penile implants.
• Prolonged erection and priapismhave occurred in patients receiving CAVERJECT. To minimize the chances of this occurring, titrate CAVERJECT IMPULSE slowly to the lowest effective dose (). Advise patients to seek immediate medical assistance for an erection that persists longer than 4 hours (2.1 Important Dosage and Administration Instructions• Alprostadil is available in different strengths and presentations. Determine the most suitable dose and presentation for each patient. Use a new syringe for each dose of CAVERJECT.• Titrate the dose of CAVERJECT IMPULSE for each patient to the lowest effective dose.• CAVERJECT IMPULSE doses greater than 60 mcg are not recommended.• Administer the first doses of CAVERJECT IMPULSE in the health care provider’s office by medically trained personnel.• Instruct the patient on proper use and assess that they are well trained in the self-injection technique prior to initiation of home use. Refer to the Patient Information and Instructions for Use.• Re-evaluate patients regularly (every 3 months or as clinically appropriate) and determine if dosage adjustments are needed.
).5.1 Prolonged Erection and PriapismProlonged erection, defined as erection lasting between 4 to 6 hours in duration, occurred in 4% of 1,861 patients treated up to 18 months in studies of CAVERJECT. The incidence of priapism (erections lasting more than 6 hours in duration) was 0.4%. In the event of an erection that persists longer than 4 hours, the patient should seek immediate medical assistance. If priapism is not treated immediately, penile tissue damage and permanent loss of potency may result.
To minimize the chances of prolonged erection or priapism, titrate CAVERJECT IMPULSE to the lowest effective dose
[seeDosage and Administration (2.1].In addition, do not use CAVERJECT IMPULSE in patients who have conditions that predispose them to priapism, such as sickle cell anemia or sickle cell trait, multiple myeloma, or leukemia[see Contraindications (4)].• Penile fibrosishas occurred in patients receiving CAVERJECT. Follow patients regularly to detect signs of penile fibrosis. Discontinue in patients who develop penile angulation or cavernosal fibrosis ().5.2 Penile FibrosisThe overall incidence of penile fibrosis reported in clinical studies with CAVERJECT was 3%. In one self-injection clinical study where duration of use was up to 18 months, the incidence of penile fibrosis was 7.8%.
Physical examination of the penis should be performed periodically to detect signs of penile fibrosis. Treatment with CAVERJECT IMPULSE should be discontinued in patients who develop penile angulation or cavernosal fibrosis.
• Hypotension- injections of CAVERJECT IMPULSE can lead to increased peripheral blood levels of alprostadil, especially in patients with significant corpora cavernosa venous leakage. Avoid use in patients with known cavernosal venous leakage ().5.3 HypotensionIntracavernous injections of CAVERJECT IMPULSE can increase peripheral blood levels of alprostadil which can result in hypotension. Avoid use of CAVERJECT IMPULSE in patients with known cavernosal venous leakage.
• Injection site bleedingmay occur in patients taking anticoagulants, such as warfarin or heparin. Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes ().5.4 Injection Site Bleeding When Used with AnticoagulantsPatients on anticoagulants, such as warfarin or heparin, may have increased propensity for injection site bleeding after intracavernosal injection with CAVERJECT IMPULSE. Compress the site of injection with an alcohol swab or sterile gauze for 5 minutes.
• Cardiovascular risk related to underlying medical conditions- Underlying treatable medical causes of erectile dysfunction should be diagnosed and treated prior to initiation of therapy ().5.5 Cardiovascular Risk Related to Underlying Medical ConditionsThere is a potential for cardiac risk of sexual activity in patients with preexisting cardiovascular disease. Therefore, treatments for erectile dysfunction, including CAVERJECT IMPULSE, generally should not be used in men for whom sexual activity is inadvisable because of their underlying cardiovascular status. In addition, the evaluation of erectile dysfunction should include a determination of potential underlying causes and the identification of appropriate treatment following a complete medical assessment.
• Risks of use in combination with other vasoactive medications injected intracavernosally- Safety and efficacy of combinations of CAVERJECT and other vasoactive agents have not been systematically studied. Use of such combinations is not recommended ().5.6 Risks of Use in Combination with Other Vasoactive Medications Injected IntracavernosallyThe safety and efficacy of combinations of CAVERJECT IMPULSE and other vasoactive agents injected intracavernosally have not been established in clinical studies. The risks of prolonged erection, priapism, and hypotension may be increased.
• Risk of needle breakage – A superfine needle is used for CAVERJECT IMPULSE and cases of needle breakage have been reported. Careful instruction in proper patient handling and injection techniques may minimize this risk ().5.7 Needle BreakageCAVERJECT IMPULSE uses a superfine (29 gauge) needle for administration. As with all superfine needles, the possibility of needle breakage exists. Needle breakage, with a portion of the needle remaining in the penis, has been reported and, in some cases, has required hospitalization and surgical removal. Careful instruction in proper patient handling and injection techniques may minimize the potential for needle breakage
[see Dosage and Administration (2.3)and Adverse Reactions (6.2)].• Benzyl alcohol– Serious and fatal adverse reactions can occur in neonates and low birth weight infants treated with benzyl alcohol-preserved formulations in infusion solutions, including CAVERJECT IMPULSE. CAVERJECT IMPULSE is not indicated in neonates and infants ().5.8 Risk of Serious Adverse Reactions in Infants due to Benzyl AlcoholThe preservative benzyl alcohol contained in CAVERJECT IMPULSE has been associated with serious adverse events, including the "gasping syndrome", and death in pediatric patients. The minimum amount of benzyl alcohol at which toxicity may occur is not known. The risk of benzyl alcohol toxicity depends on the quantity administered and the liver and kidneys' capacity to detoxify the chemical. Premature and low-birth weight infants may be more likely to develop toxicity. CAVERJECT IMPULSE is not indicated for use in pediatric patients.
• Counsel patients about sexually transmitted diseases. Counsel patients about the protective measures necessary to guard against sexually transmitted disease including the Human Immunodeficiency Virus (HIV) ().5.9 Counsel Patients About Sexually Transmitted DiseasesThe use of CAVERJECT IMPULSE offers no protection against sexually transmitted diseases. Counsel patients about the protective measures necessary to guard against sexually transmitted diseases, including the Human Immunodeficiency Virus (HIV).