Cefaclor
Cefaclor Prescribing Information
Cefaclor is indicated in the treatment of the following infections when caused by susceptible strains of the designated microorganisms:
Appropriate culture and susceptibility studies should be performed to determine susceptibility of the causative organism to cefaclor.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefaclor for Oral Suspension and other antibacterial drugs, Cefaclor for Oral Suspension should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefaclor is administered orally.
Cefaclor for Oral Suspension, USP | ||
20 mg/kg/day | ||
Weight | 125 mg/5 mL | 250 mg/5 mL |
| 9 kg | 1/2 tsp t.i.d. | |
| 18 kg | 1 tsp t.i.d. | 1/2 tsp t.i.d. |
40 mg/kg/day | ||
| 9 kg | 1 tsp t.i.d. | 1/2 tsp t.i.d. |
| 18 kg | 1 tsp t.i.d. | |
Cefaclor for Oral Suspension, USP | |
20 mg/kg/day (Pharyngitis) | |
Weight | 375 mg/5 mL |
| 18 kg | 1/2 tsp b.i.d. |
40 mg/kg/day (Otitis Media) | |
| 9 kg | 1/2 tsp b.i.d. |
| 18 kg | 1 tsp b.i.d. |
Cefaclor may be administered in the presence of impaired renal function. Under such a condition, the dosage usually is unchanged (see
Prescribing cefaclor in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increase the risk of the development of drug- resistant bacteria.
Prolonged use of cefaclor may result in the overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Positive direct Coombs' tests have been reported during treatment with the cephalosporin antibiotics. It should be recognized that a positive Coombs' test may be due to the drug, e.g., in hematologic studies or in transfusion cross-matching procedures when antiglobulin tests are performed on the minor side or in Coombs' testing of newborns whose mothers have received cephalosporin antibiotics before parturition.
Cefaclor should be administered with caution in the presence of markedly impaired renal function. Since the half-life of cefaclor in anuria is 2.3 to 2.8 hours, dosage adjustments for patients with moderate or severe renal impairment are usually not required. Clinical experience with cefaclor under such conditions is limited; therefore, careful clinical observation and laboratory studies should be made.
As with other β-lactam antibiotics, the renal excretion of cefaclor is inhibited by probenecid.
Antibiotics, including cephalosporins, should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Patients should be counseled that antibacterial drugs including Cefaclor for Oral Suspension should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefaclor for Oral Suspension is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping dose or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefaclor for Oral Suspension or other antibacterial drugs in the future.
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Patients receiving cefaclor may show a false-positive reaction for glucose in the urine with tests that use Benedict's and Fehling's solutions and also with Clinitest®tablets.
There have been reports of increased anticoagulant effect when cefaclor and oral anticoagulants were administered concomitantly.
Studies have not been performed to determine potential for carcinogenicity, mutagenicity, or impairment of fertility.
Reproduction studies have been performed in mice and rats at doses up to 12 times the human dose and in ferrets given 3 times the maximum human dose and have revealed no harm to the fetus due to cefaclor. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
The effect of cefaclor on labor and delivery is unknown.
Small amounts of cefaclor have been detected in mother's milk following administration of single 500 mg doses. Average levels were 0.18, 0.20, 0.21, and 0.16 mcg/mL at 2, 3, 4, and 5 hours, respectively. Trace amounts were detected at 1 hour. The effect on nursing infants is not known. Caution should be exercised when cefaclor is administered to a nursing woman.
Safety and effectiveness of this product for use in infants less than 1 month of age have not been established.
Of the 3,703 patients in clinical studies of cefaclor, 594 (16.0%) were 65 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney (see
In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cefaclor should be administered for at least 10 days.
Add appropriate water volume as indicated in the following table in two portions to dry mixture in the bottle. Shake well after each addition.
Each 5 mL (approximately one teaspoonful) will then contain Cefaclor, USP, monohydrate equivalent to 250 mg anhydrous cefaclor, respectively, as shown in the following table.
Oversize bottle provides extra space for shaking.
Cefaclor For Oral Suspension, USP | ||
Strength Package Size (when mixed) | Water Volume to Add | Anhydrous Cefaclor/5 mL (approx. one teaspoonful) |
250 mg/5 Ml 150 mL | 106 mL | 250 mg |
Cefaclor is contraindicated in patients with known allergy to the cephalosporin group of antibiotics.
Adverse effects considered to be related to therapy with cefaclor are listed below:
Cases of
More severe hypersensitivity reactions, including Stevens-Johnson syndrome, toxic epidermal necrolysis, and anaphylaxis have been reported rarely.
Anaphylactoid events may be manifested by solitary symptoms, including angioedema, asthenia, edema (including face and limbs), dyspnea, paresthesias, syncope, hypotension, or vasodilatation. Anaphylaxis may be more common in patients with a history of penicillin allergy.
Rarely, hypersensitivity symptoms may persist for several months.
Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment. (see
Antibiotics, including cefaclor, should be administered cautiously to any patient who has demonstrated some form of allergy, particularly to drugs.
Transitory abnormalities in clinical laboratory test results have been reported. Although they were of uncertain etiology, they are listed below to serve as alerting information for the physician.
There have been rare reports of increased prothrombin time with or without clinical bleeding in patients receiving cefaclor and Coumadin® concomitantly.
In addition to the adverse reactions listed above that have been observed in patients treated with cefaclor, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics: fever, abdominal pain, superinfection, renal dysfunction, toxic nephropathy, hemorrhage, false-positive test for urinary glucose, elevated bilirubin, elevated LDH, and pancytopenia.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced. If seizures associated with drug therapy occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated (see
Cefaclor is administered orally.
Cefaclor for Oral Suspension, USP | ||
20 mg/kg/day | ||
Weight | 125 mg/5 mL | 250 mg/5 mL |
| 9 kg | 1/2 tsp t.i.d. | |
| 18 kg | 1 tsp t.i.d. | 1/2 tsp t.i.d. |
40 mg/kg/day | ||
| 9 kg | 1 tsp t.i.d. | 1/2 tsp t.i.d. |
| 18 kg | 1 tsp t.i.d. | |
Cefaclor for Oral Suspension, USP | |
20 mg/kg/day (Pharyngitis) | |
Weight | 375 mg/5 mL |
| 18 kg | 1/2 tsp b.i.d. |
40 mg/kg/day (Otitis Media) | |
| 9 kg | 1/2 tsp b.i.d. |
| 18 kg | 1 tsp b.i.d. |
Cefaclor may be administered in the presence of impaired renal function. Under such a condition, the dosage usually is unchanged (see
In the treatment of β-hemolytic streptococcal infections, a therapeutic dosage of cefaclor should be administered for at least 10 days.
Add appropriate water volume as indicated in the following table in two portions to dry mixture in the bottle. Shake well after each addition.
Each 5 mL (approximately one teaspoonful) will then contain Cefaclor, USP, monohydrate equivalent to 250 mg anhydrous cefaclor, respectively, as shown in the following table.
Oversize bottle provides extra space for shaking.
Cefaclor For Oral Suspension, USP | ||
Strength Package Size (when mixed) | Water Volume to Add | Anhydrous Cefaclor/5 mL (approx. one teaspoonful) |
250 mg/5 Ml 150 mL | 106 mL | 250 mg |
Unless 5 times the normal dose of cefaclor has been ingested, gastrointestinal decontamination will not be necessary.
Protect the patient's airway and support ventilation and perfusion. Meticulously monitor and maintain, within acceptable limits, the patient's vital signs, blood gases, serum electrolytes, etc. Absorption of drugs from the gastrointestinal tract may be decreased by giving activated charcoal, which, in many cases, is more effective than emesis or lavage; consider charcoal instead of or in addition to gastric emptying. Repeated doses of charcoal over time may hasten elimination of some drugs that have been absorbed. Safeguard the patient's airway when employing gastric emptying or charcoal.
Forced diuresis, peritoneal dialysis, hemodialysis, or charcoal hemoperfusion have not been established as beneficial for an overdose of cefaclor.
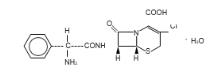
Cefaclor, USP, is a semisynthetic cephalosporin antibiotic for oral administration. It is chemically designated as 3-chloro-7-D-(2-phenylglycinamido) -3-cephem-4-carboxylic acid monohydrate. The chemical formula for cefaclor is C15H14ClN3O4S•H2O and the molecular weight is 385.82.
After mixing, each 5 mL of Cefaclor for Oral Suspension will contain cefaclor monohydrate equivalent to 250 mg (0.68 mmol) anhydrous cefaclor. The suspensions also contain methylcellulose, sodium lauryl sulfate, sucrose, and xanthan gum, FD&C Red No. 40, strawberry flavor.
The color of drug powder in the dry powder state is white to off-white. After reconstitution, it turns to a red suspension.
Cefaclor is well-absorbed after oral administration to fasting subjects. Total absorption is the same whether the drug is given with or without food; however, when it is taken with food, the peak concentration achieved is 50% to 75% of that observed when the drug is administered to fasting subjects and generally appears from three-fourths to 1 hour later. Following administration of 250 mg, 500 mg, and 1 g doses to fasting subjects, average peak serum levels of approximately 7, 13, and 23 mcg/mL, respectively, were obtained within 30 to 60 minutes. Approximately 60% to 85% of the drug is excreted unchanged in the urine within 8 hours, the greater portion being excreted within the first 2 hours. During this 8-hour period, peak urine concentrations following the 250 mg, 500 mg and 1 g doses were approximately 600, 900 and 1,900 mcg/mL, respectively. The serum half-life in normal subjects is 0.6 to 0.9 hour. In patients with reduced renal function, the serum half-life of cefaclor is slightly prolonged. In those with complete absence of renal function, the plasma half-life of the intact molecule is 2.3 to 2.8 hours. Excretion pathways in patients with markedly impaired renal function have not been determined. Hemodialysis shortens the half-life by 25% to 30%.