Cefprozil
Cefprozil Prescribing Information
Cefprozil is indicated for the treatment of patients with mild to moderate infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
UPPER RESPIRATORY TRACT
NOTE: The usual drug of choice in the treatment and prevention of streptococcal infections, including the prophylaxis of rheumatic fever, is penicillin given by the intramuscular route. Cefprozil is generally effective in the eradication of
NOTE: In the treatment of otitis media due to β-lactamase producing organisms, cefprozil had bacteriologic eradication rates somewhat lower than those observed with a product containing a specific β-lactamase inhibitor. In considering the use of cefprozil, lower overall eradication rates should be balanced against the susceptibility patterns of the common microbes in a given geographic area and the increased potential for toxicity with products containing β-lactamase inhibitors.
LOWER RESPIRATORY TRACT
SKIN AND SKIN STRUCTURE
To reduce the development of drug-resistant bacteria and maintain the effectiveness of cefprozil and other antibacterial drugs, cefprozil should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Cefprozil is administered orally.
Population/Infection | Dosage (mg) | Duration (days) |
ADULTS (13 years and older) UPPER RESPIRATORY TRACT | ||
Pharyngitis/Tonsillitis | 500 q24h | 10a |
Acute Sinusitis | 250 q12h or | 10 |
(For moderate to severe infections, the higher dose should be used) | 500 q12h | |
LOWER RESPIRATORY TRACT | ||
Acute Bacterial Exacerbation of Chronic Bronchitis | 500 q12h | 10 |
SKIN AND SKIN STRUCTURE | ||
Uncomplicated Skin and Skin Structure Infections | 250 q12h or 500 q24h or 500 q12h | 10 |
CHILDREN (2 years–12 years) UPPER RESPIRATORY TRACTb | ||
Pharyngitis/Tonsillitis SKIN AND SKIN STRUCTURE | 7.5 mg/kg q12h | 10a |
Uncomplicated Skin and Skin Structure Infections | 20 mg/kg q24h | 10 |
INFANTS & CHILDREN (6 months–12 years) UPPER RESPIRATORY TRACTb | ||
Otitis Media (See INDICATIONS AND USAGE and CLINICAL STUDIES) | 15 mg/kg q12h | 10 |
Acute Sinusitis | 7.5 mg/kg q12h or | 10 |
(For moderate to severe infections, the higher dose should be used) | 15 mg/kg q12h | |
a In the treatment of infections due to Streptococcus pyogenes, cefprozil should be administered for at least 10 days.
b Not to exceed recommended adult doses.
Creatinine Clearance (mL/min) | Dosage (mg) | Dosing Interval |
30–120 | standard | standard |
0–29* | 50% of standard | standard |
*Cefprozil is in part removed by hemodialysis; therefore, cefprozil should be administered after the completion of hemodialysis.
Hepatic Impairment
No dosage adjustment is necessary for patients with impaired hepatic function.
Cefprozil is contraindicated in patients with known allergy to the cephalosporin class of antibiotics.
The adverse reactions to cefprozil are similar to those observed with other orally administered cephalosporins. Cefprozil was usually well tolerated in controlled clinical trials. Approximately 2% of patients discontinued cefprozil therapy due to adverse events.
The most common adverse effects observed in patients treated with cefprozil are:
The following adverse events, regardless of established causal relationship to cefprozil, have been rarely reported during postmarketing surveillance: anaphylaxis, angioedema, colitis (including pseudomembranous colitis), erythema multiforme, fever, serum-sickness like reactions, Stevens-Johnson syndrome, and thrombocytopenia.
In addition to the adverse reactions listed above which have been observed in patients treated with cefprozil, the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Aplastic anemia, hemolytic anemia, hemorrhage, renal dysfunction, toxic epidermal necrolysis, toxic nephropathy, prolonged prothrombin time, positive Coombs’ test, elevated LDH, pancytopenia, neutropenia, agranulocytosis.
Several cephalosporins have been implicated in triggering seizures, particularly in patients with renal impairment, when the dosage was not reduced. (See
Nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporin antibiotics. Concomitant administration of probenecid doubled the AUC for cefprozil.
The bioavailability of the capsule formulation of cefprozil was not affected when administered 5 minutes following an antacid.
Cefprozil is a semi-synthetic broad-spectrum cephalosporin antibiotic.
Cefprozil is a cis and trans isomeric mixture (≥90% cis). The chemical name for the monohydrate is (6R,7R)-7-[(R)-2-Amino-2-(p-hydroxyphenyl)acetamido]-8-oxo-3-propenyl-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate, and the structural formula is:
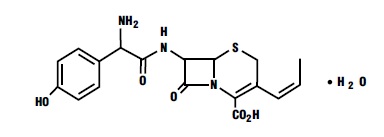
Cefprozil is a white to yellowish powder with a molecular formula for the monohydrate of C18H19N3O5S•H2O and a molecular weight of 407.45.
Cefprozil tablets are intended for oral administration.
Cefprozil tablets contain cefprozil equivalent to 250 mg or 500 mg of anhydrous cefprozil. In addition, each tablet contains the following inactive ingredients: microcrystalline cellulose, sodium starch glycolate, magnesium stearate, Hypromellose 2910, polyethylene glycol 400, titanium dioxide, and yellow iron oxide.