Ceftriaxone
Ceftriaxone Prescribing Information
Before instituting treatment with Ceftriaxone for Injection, USP, appropriate specimens should be obtained for isolation of the causative organism and for determination of its susceptibility to the drug. Therapy may be instituted prior to obtaining results of susceptibility testing.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ceftriaxone for Injection, USP and other antibacterial drugs, Ceftriaxone for Injection, USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ceftriaxone for Injection, USP is indicated for the treatment of the following infections when caused by susceptible organisms:
NOTE: In one study lower clinical cure rates were observed with a single dose of Ceftriaxone for Injection, USP compared to 10 days of oral therapy. In a second study comparable cure rates were observed between single dose Ceftriaxone for Injection, USP and the comparator. The potentially lower clinical cure rate of Ceftriaxone for Injection, USP should be balanced against the potential advantages of parenteral therapy (see CLINICAL STUDIES).
*Efficacy for this organism in this organ system was studied in fewer than ten infections.
When administered prior to surgical procedures for which it is indicated, a single 1 g dose of Ceftriaxone for Injection, USP provides protection from most infections due to susceptible organisms throughout the course of the procedure.
Ceftriaxone may be administered intravenously or intramuscularly.
Do not use diluents containing calcium, such as Ringer’s solution or Hartmann’s solution, to reconstitute ceftriaxone vials or to further dilute a reconstituted vial for IV administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same IV administration line. Ceftriaxone must not be administered simultaneously with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid (see WARNINGS).
There have been no reports of an interaction between ceftriaxone and oral calcium-containing products or interaction between intramuscular ceftriaxone and calcium-containing products (IV or oral).
Ceftriaxone is contraindicated in patients with known hypersensitivity to ceftriaxone, any of its excipients or to any other cephalosporin. Patients with previous hypersensitivity reactions to penicillin and other beta lactam antibacterial agents may be at greater risk of hypersensitivity to ceftriaxone (see WARNINGS – Hypersensitivity).
Ceftriaxone is contraindicated in neonates (≤ 28 days) if they require (or are expected to require) treatment with calcium-containing IV solutions, including continuous calcium-containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium (see
Cases of fatal outcomes in which a crystalline material was observed in the lungs and kidneys at autopsy have been reported in neonates receiving ceftriaxone and calcium-containing fluids.
In some of these cases, the same intravenous infusion line was used for both ceftriaxone and calcium-containing fluids and in some a precipitate was observed in the intravenous infusion line. There have been no similar reports in patients other than neonates.
Intravenous administration of ceftriaxone solutions containing lidocaine is contraindicated. When lidocaine solution is used as a solvent with ceftriaxone for intramuscular injection, exclude all contraindications to lidocaine. Refer to the prescribing information of lidocaine.
Ceftriaxone is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to ceftriaxone therapy or of uncertain etiology, were observed:
Other rarely observed adverse reactions (< 0.1%) include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
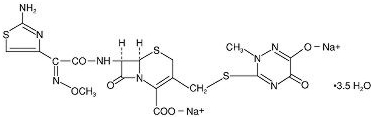
Ceftriaxone for Injection, USP is a sterile, semisynthetic, broad-spectrum cephalosporin antibiotic for intravenous or intramuscular administration. Ceftriaxone sodium is (6
The chemical formula of ceftriaxone sodium is C18H16N8Na2O7S3·3.5H2O. It has a calculated molecular weight of 661.60 and the following structural formula:
Ceftriaxone for Injection, USP is a white to yellowish-orange crystalline powder which is readily soluble in water, sparingly soluble in methanol and very slightly soluble in ethanol. The pH of a 1% aqueous solution is approximately 6.7. The color of Ceftriaxone for Injection, USP solutions ranges from light yellow to amber, depending on the length of storage, concentration and diluent used.
Ceftriaxone for Injection, USP contains approximately 83 mg (3.6 mEq) of sodium per gram of ceftriaxone activity.
Average plasma concentrations of ceftriaxone following a single 30-minute intravenous (IV) infusion of a 0.5, 1 or 2 g dose and intramuscular (IM) administration of a single 0.5 (250 mg/mL or 350 mg/mL concentrations) or 1 g dose in healthy subjects are presented in Table 1.
Dose/Route | Average Plasma Concentrations (mcg/mL) | ||||||||
0.5 hr | 1 hr | 2 hr | 4 hr | 6 hr | 8 hr | 12 hr | 16 hr | 24 hr | |
0.5 g IVIV doses were infused at a constant rate over 30 minutes. | 82 | 59 | 48 | 37 | 29 | 23 | 15 | 10 | 5 |
0.5 g IM 250 mg/mL | 22 | 33 | 38 | 35 | 30 | 26 | 16 | ND | 5 |
0.5 g IM 350 mg/mL | 20 | 32 | 38 | 34 | 31 | 24 | 16 | ND | 5 |
1 g IV | 151 | 111 | 88 | 67 | 53 | 43 | 28 | 18 | 9 |
1 g IM | 40 | 68 | 76 | 68 | 56 | 44 | 29 | ND | ND |
2 g IV | 257 | 192 | 154 | 117 | 89 | 74 | 46 | 31 | 15 |
ND = Not determined.
Ceftriaxone was completely absorbed following IM administration with mean maximum plasma concentrations occurring between 2 and 3 hours post-dose. Multiple IV or IM doses ranging from 0.5 to 2 g at 12- to 24-hour intervals resulted in 15% to 36% accumulation of ceftriaxone above single dose values.
Ceftriaxone concentrations in urine are shown in Table 2.
Dose/Route | Average Urinary Concentrations (mcg/mL) | |||||
0 to 2 hr | 2 to 4 hr | 4 to 8 hr | 8 to 12 hr | 12 to 24 hr | 24 to 48 hr | |
0.5 g IV | 526 | 366 | 142 | 87 | 70 | 15 |
0.5 g IM | 115 | 425 | 308 | 127 | 96 | 28 |
1 g IV | 995 | 855 | 293 | 147 | 132 | 32 |
1 g IM | 504 | 628 | 418 | 237 | ND | ND |
2 g IV | 2692 | 1976 | 757 | 274 | 198 | 40 |
ND = Not determined.
Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug and the remainder was secreted in the bile and ultimately found in the feces as microbiologically inactive compounds. After a 1 g IV dose, average concentrations of ceftriaxone, determined from 1 to 3 hours after dosing, were 581 mcg/mL in the gallbladder bile, 788 mcg/mL in the common duct bile, 898 mcg/mL in the cystic duct bile, 78.2 mcg/g in the gallbladder wall and 62.1 mcg/mL in the concurrent plasma.
Over a 0.15 to 3 g dose range in healthy adult subjects, the values of elimination half-life ranged from 5.8 to 8.7 hours; apparent volume of distribution from 5.78 to 13.5 L; plasma clearance from 0.58 to 1.45 L/hour; and renal clearance from 0.32 to 0.73 L/hour. Ceftriaxone is reversibly bound to human plasma proteins, and the binding decreased from a value of 95% bound at plasma concentrations of < 25 mcg/mL to a value of 85% bound at 300 mcg/mL. Ceftriaxone crosses the blood placenta barrier.
The average values of maximum plasma concentration, elimination half-life, plasma clearance and volume of distribution after a 50 mg/kg IV dose and after a 75 mg/kg IV dose in pediatric patients suffering from bacterial meningitis are shown in Table 3. Ceftriaxone penetrated the inflamed meninges of infants and pediatric patients; CSF concentrations after a 50 mg/kg IV dose and after a 75 mg/kg IV dose are also shown in Table 3.
50 mg/kg IV | 75 mg/kg IV | |
Maximum Plasma Concentrations (mcg/mL) | 216 | 275 |
Elimination Half-life (hr) | 4.6 | 4.3 |
Plasma Clearance (mL/hr/kg) | 49 | 60 |
Volume of Distribution (mL/kg) | 338 | 373 |
CSF Concentration—inflamed meninges (mcg/mL) | 5.6 | 6.4 |
Range (mcg/mL) | 1.3 to 18.5 | 1.3 to 44 |
Time after dose (hr) | 3.7 (± 1.6) | 3.3 (± 1.4) |
Compared to that in healthy adult subjects, the pharmacokinetics of ceftriaxone were only minimally altered in elderly subjects and in patients with renal impairment or hepatic dysfunction (Table 4); therefore, dosage adjustments are not necessary for these patients with ceftriaxone dosages up to 2 g per day. Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis; in six of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced.
Subject Group | Elimination Half-Life (hr) | Plasma Clearance (L/hr) | Volume of Distribution (L) |
Healthy Subjects | 5.8 to 8.7 | 0.58 to 1.45 | 5.8 to 13.5 |
Elderly Subjects (mean age, 70.5 yr) | 8.9 | 0.83 | 10.7 |
Patients With Renal Impairment | |||
Hemodialysis Patients (0 to 5 mL/min)Creatinine clearance. | 14.7 | 0.65 | 13.7 |
Severe (5 to 15 mL/min) | 15.7 | 0.56 | 12.5 |
Moderate (16 to 30 mL/min) | 11.4 | 0.72 | 11.8 |
Mild (31 to 60 mL/min) | 12.4 | 0.70 | 13.3 |
Patients With Liver Disease | 8.8 | 1.1 | 13.6 |
The elimination of ceftriaxone is not altered when ceftriaxone is co-administered with probenecid.