Ceftriaxone
Ceftriaxone Prescribing Information
Before instituting treatment with Ceftriaxone for Injection, appropriate specimens should be obtained for isolation of the causative organism and for determination of its susceptibility to the drug. Therapy may be instituted prior to obtaining results of susceptibility testing.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ceftriaxone for Injection and other antibacterial drugs, Ceftriaxone for Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Ceftriaxone for Injection is indicated for the treatment of the following infections when caused by susceptible organisms:
Ceftriaxone for injection may be administered intravenously or intramuscularly. However, the intent of this Pharmacy Bulk Package is for the preparation of solutions for intravenous infusion only. Ceftriaxone for injection should be administered intravenously by infusion over a period of 30 minutes.
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone for injection bottles or to further dilute a reconstituted bottle for intravenous administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone for injection is mixed with calcium-containing solutions in the same intravenous administration line.
Ceftriaxone for injection must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-Site. However, in patients other than neonates, ceftriaxone for injection and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid (see
Before therapy with ceftriaxone is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cephalosporins, penicillins and other beta-lactam agents or other drugs. This product should be given cautiously to penicillin and other beta-lactam agent-sensitive patients. Antibacterial drugs should be administered with caution to any patient who has demonstrated some form of allergy, particularly to drugs. Serious acute hypersensitivity reactions may require the use of subcutaneous epinephrine and other emergency measures.
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions (i.e., anaphylaxis) have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated.
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone bottles or to further dilute a reconstituted bottle for intravenous administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same intravenous administration line. Ceftriaxone must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid.
Serious neurological adverse reactions have been reported during postmarketing surveillance with ceftriaxone use. These reactions include encephalopathy (disturbance of consciousness including somnolence, lethargy, and confusion), seizures, myoclonus, and non-convulsive status epilepticus (see
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia while on ceftriaxone, the diagnosis of a cephalosporin associated anemia should be considered and ceftriaxone stopped until the etiology is determined.
There have been no reports of an interaction between ceftriaxone for injection and oral calcium-containing products or interaction between intramuscular ceftriaxone for injection and calcium-containing products (intravenous or oral).
Ceftriaxone for injection is contraindicated in patients with known hypersensitivity to ceftriaxone, any of its excipients or to any other cephalosporin. Patients with previous hypersensitivity reactions to penicillin and other beta lactam antibacterial agents may be at greater risk of hypersensitivity to ceftriaxone (see
Before therapy with ceftriaxone is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cephalosporins, penicillins and other beta-lactam agents or other drugs. This product should be given cautiously to penicillin and other beta-lactam agent-sensitive patients. Antibacterial drugs should be administered with caution to any patient who has demonstrated some form of allergy, particularly to drugs. Serious acute hypersensitivity reactions may require the use of subcutaneous epinephrine and other emergency measures.
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions (i.e., anaphylaxis) have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated.
Ceftriaxone is generally well tolerated. In clinical trials, the following adverse reactions, which were considered to be related to ceftriaxone therapy or of uncertain etiology, were observed:
Pain, induration and tenderness was 1% overall. Phlebitis was reported in <1% after intravenous administration. The incidence of warmth, tightness or induration was 17% (3/17) after intramuscular administration of 350 mg/mL and 5% (1/20) after intramuscular administration of 250 mg/mL.
Injection site pain (0.6%).
Rash (1.7%). Less frequently reported (<1%) were pruritus, fever or chills.
Genital fungal infection (0.1%).
Eosinophilia (6%), thrombocytosis (5.1%) and leukopenia (2.1%). Less frequently reported (<1%) were anemia, hemolytic anemia, neutropenia, lymphopenia, thrombocytopenia and prolongation of the prothrombin time.
Granulocytopenia (0.9%), coagulopathy (0.4%).
Diarrhea/loose stools (2.7%). Less frequently reported (<1%) were nausea or vomiting, and dysgeusia. The onset of pseudomembranous colitis symptoms may occur during or after antibacterial treatment (see
Before therapy with ceftriaxone is instituted, careful inquiry should be made to determine whether the patient has had previous hypersensitivity reactions to cephalosporins, penicillins and other beta-lactam agents or other drugs. This product should be given cautiously to penicillin and other beta-lactam agent-sensitive patients. Antibacterial drugs should be administered with caution to any patient who has demonstrated some form of allergy, particularly to drugs. Serious acute hypersensitivity reactions may require the use of subcutaneous epinephrine and other emergency measures.
As with all beta-lactam antibacterial agents, serious and occasionally fatal hypersensitivity reactions (i.e., anaphylaxis) have been reported. In case of severe hypersensitivity reactions, treatment with ceftriaxone must be discontinued immediately and adequate emergency measures must be initiated.
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone bottles or to further dilute a reconstituted bottle for intravenous administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone is mixed with calcium-containing solutions in the same intravenous administration line. Ceftriaxone must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-site. However, in patients other than neonates, ceftriaxone and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid.
Serious neurological adverse reactions have been reported during postmarketing surveillance with ceftriaxone use. These reactions include encephalopathy (disturbance of consciousness including somnolence, lethargy, and confusion), seizures, myoclonus, and non-convulsive status epilepticus (see
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against
An immune mediated hemolytic anemia has been observed in patients receiving cephalosporin class antibacterials including ceftriaxone. Severe cases of hemolytic anemia, including fatalities, have been reported during treatment in both adults and children. If a patient develops anemia while on ceftriaxone, the diagnosis of a cephalosporin associated anemia should be considered and ceftriaxone stopped until the etiology is determined.
Elevations of aspartate aminotransferase (AST) (3.1%) or alanine aminotransferase (ALT) (3.3%). Less frequently reported (<1%) were elevations of alkaline phosphatase and bilirubin.
Elevations of the BUN (1.2%). Less frequently reported (<1%) were elevations of creatinine and the presence of casts in the urine.
Headache or dizziness were reported occasionally (<1%).
Moniliasis or vaginitis were reported occasionally (<1%).
Diaphoresis and flushing were reported occasionally (<1%).
Blood creatinine increased (0.6%).
Other rarely observed adverse reactions (<0.1%) include abdominal pain, agranulocytosis, allergic pneumonitis, anaphylaxis, basophilia, biliary lithiasis, bronchospasm, colitis, dyspepsia, epistaxis, flatulence, gallbladder sludge, glycosuria, hematuria, jaundice, leukocytosis, lymphocytosis, monocytosis, nephrolithiasis, palpitations, a decrease in the prothrombin time, renal precipitations, seizures, and serum sickness.
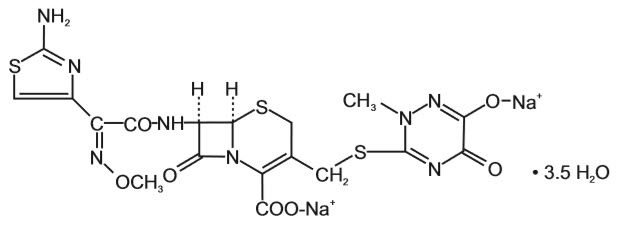
Ceftriaxone for Injection, USP is a sterile, semisynthetic, broad-spectrum cephalosporin antibiotic for intravenous or intramuscular administration. Ceftriaxone sodium is (6
The chemical formula of ceftriaxone sodium is C18H16N8Na2O7S3∙3.5H2O. It has a calculated molecular weight of 661.60 and the following structural formula:
Ceftriaxone sodium is a white to yellowish-orange crystalline powder which is readily soluble in water, sparingly soluble in methanol and very slightly soluble in ethanol. The pH of a 1% aqueous solution is approximately 6.7. The color of Ceftriaxone sodium solutions ranges from light yellow to amber, depending on the length of storage, concentration and diluent used.
Each Pharmacy Bulk Package is supplied as a dry powder in Pharmacy Bulk Package bottles containing sterile ceftriaxone sodium, USP equivalent to 10 grams of ceftriaxone and is intended for intravenous infusion only. Ceftriaxone sodium contains approximately 83 mg (3.6 mEq) of sodium per gram of ceftriaxone activity.
A Pharmacy Bulk Package is a container of a sterile preparation for parenteral use that contains many single doses. The contents are intended for use in a pharmacy admixture program and are restricted to the preparation of admixtures for intravenous infusion. FURTHER DILUTION IS REQUIRED BEFORE USE. (See
Ceftriaxone for injection may be administered intravenously or intramuscularly. However, the intent of this Pharmacy Bulk Package is for the preparation of solutions for intravenous infusion only. Ceftriaxone for injection should be administered intravenously by infusion over a period of 30 minutes.
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone for injection bottles or to further dilute a reconstituted bottle for intravenous administration because a precipitate can form. Precipitation of ceftriaxone-calcium can also occur when ceftriaxone for injection is mixed with calcium-containing solutions in the same intravenous administration line.
Ceftriaxone for injection must not be administered simultaneously with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition via a Y-Site. However, in patients other than neonates, ceftriaxone for injection and calcium-containing solutions may be administered sequentially of one another if the infusion lines are thoroughly flushed between infusions with a compatible fluid (see
There have been no reports of an interaction between ceftriaxone for injection and oral calcium-containing products or interaction between intramuscular ceftriaxone for injection and calcium-containing products (intravenous or oral).
Hyperbilirubinemic neonates, especially prematures, should not be treated with ceftriaxone for injection. Ceftriaxone for injection is contraindicated in premature neonates (see
Ceftriaxone for injection is contraindicated in neonates (≤ 28 days) if they require (or are expected to require) treatment with calcium-containing intravenous solutions, including continuous calcium-containing infusions such as parenteral nutrition because of the risk of precipitation of ceftriaxone-calcium (see
Intravenous doses should be given over 60 minutes in neonates to reduce the risk of bilirubin encephalopathy.
For the treatment of skin and skin structure infections, the recommended total daily dose is 50 to 75 mg/kg given once a day (or in equally divided doses twice a day). The total daily dose should not exceed 2 grams.
For the treatment of serious miscellaneous infections other than meningitis, the recommended total daily dose is 50 to 75 mg/kg, given in divided doses every 12 hours. The total daily dose should not exceed 2 grams.
In the treatment of meningitis, it is recommended that the initial therapeutic dose be 100 mg/kg (not to exceed 4 grams). Thereafter, a total daily dose of 100 mg/kg/day (not to exceed 4 grams daily) is recommended. The daily dose may be administered once a day (or in equally divided doses every 12 hours). The usual duration of therapy is 7 to 14 days.
The usual adult daily dose is 1 to 2 grams given once a day (or in equally divided doses twice a day) depending on the type and severity of infection. The total daily dose should not exceed 4 grams.
If
For preoperative use (surgical prophylaxis), a single dose of 1 gram administered intravenously 1/2 to 2 hours before surgery is recommended.
Generally, ceftriaxone for injection therapy should be continued for at least 2 days after the signs and symptoms of infection have disappeared. The usual duration of therapy is 4 to 14 days; in complicated infections, longer therapy may be required.
When treating infections caused by
No dosage adjustment is necessary for patients with impairment of renal or hepatic function (see
The dosages recommended for adults require no modification in elderly patients, up to 2 g per day, provided there is no severe renal and hepatic impairment (see
| PHARMACY BULK PACKAGE NOT FOR DIRECT INFUSION |
The 10 gram bottle should be reconstituted with 95 mL of an appropriate intravenous diluent in a suitable work area such as a laminar flow hood. The resulting solution will contain approximately 100 mg per mL of ceftriaxone.
The container closure may be penetrated only one time, utilizing a suitable sterile transfer device or dispensing set which allows measured distribution of the contents. (A sterile substance which must be reconstituted prior to use may require a separate closure entry.) Use of this product is restricted to a suitable work area, such as a laminar flow hood.
The withdrawal of container contents should be accomplished without delay. However, should this not be possible, a maximum time of
Unused portions of solution held longer than the recommended time periods should be discarded.
RECONSTITUTED BULK SOLUTIONS SHOULD NOT BE USED FOR DIRECT INFUSION. |
Transfer individual dose to appropriate intravenous solutions as soon as possible following reconstitution of the bulk package. The stability of the solution that has been transferred into a container varies according to diluent, concentration and temperature (see
Do not use diluents containing calcium, such as Ringer's solution or Hartmann's solution, to reconstitute ceftriaxone for injection bottles or to further dilute a reconstituted bottle for intravenous administration. Particulate formation can result.
Ceftriaxone for injection has been shown to be compatible with Flagyl®IV (metronidazole hydrochloride). The concentration should not exceed 5 to 7.5 mg per mL metronidazole hydrochloride with ceftriaxone 10 mg per mL as an admixture. The admixture is stable for 24 hours at room temperature only in 0.9% sodium chloride injection or 5% dextrose in water (D5W). No compatibility studies have been conducted with the Flagyl®IV RTU®(metronidazole) formulation or using other diluents. Metronidazole at concentrations greater than 8 mg per mL will precipitate. Do not refrigerate the admixture as precipitation will occur.
Vancomycin, amsacrine, aminoglycosides, and fluconazole are incompatible with ceftriaxone in admixtures. When any of these drugs are to be administered concomitantly with ceftriaxone by intermittent intravenous infusion, it is recommended that they be given sequentially, with thorough flushing of the intravenous lines (with one of the compatible fluids) between the administrations.
Ceftriaxone for injection solutions should
Ceftriaxone for injection sterile powder should be stored at room temperature 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature] or below and protected from light. After reconstitution, protection from normal light is not necessary. The color of solutions ranges from light yellow to amber, depending on the length of storage, concentration and diluent used.
Ceftriaxone
| Diluent | Storage | ||
|---|---|---|---|
| Room Temp. (25°C) | Refrigerated (4°C) | ||
| Sterile Water | 2 days | 10 days | |
| 0.9% Sodium Chloride Solution | 2 days | 10 days | |
| 5% Dextrose Solution | 2 days | 10 days | |
| 10% Dextrose Solution | 2 days | 10 days | |
| 5% Dextrose + 0.9% Sodium Chloride SolutionData available for 10 to 40 mg per mL concentrations in this diluent in PVC containers only. | 2 days | Incompatible | |
| 5% Dextrose + 0.45% Sodium Chloride Solution | 2 days | Incompatible | |
The following
After the indicated stability time periods, unused portions of solutions should be discarded.
NOTE: Parenteral drug products should be inspected visually for particulate matter before administration.
Ceftriaxone reconstituted with 5% Dextrose or 0.9% Sodium Chloride solution at concentrations between 10 mg per mL and 40 mg per mL, and then stored in frozen state (-20°C) in PVC or polyolefin containers, remains stable for 26 weeks.
Frozen solutions of ceftriaxone for injection should be thawed at room temperature before use. After thawing, unused portions should be discarded.
| PHARMACY BULK PACKAGE NOT FOR DIRECT INFUSION |
Average plasma concentrations of ceftriaxone following a single 30-minute intravenous infusion of a 0.5, 1 or 2 g dose and intramuscular administration of a single 0.5 (250 mg/mL or 350 mg/mL concentrations) or 1 g dose in healthy subjects are presented in Table 1.
| Dose/Route | Average Plasma Concentrations (mcg/mL) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0.5 hr | 1 hr | 2 hr | 4 hr | 6 hr | 8 hr | 12 hr | 16 hr | 24 hr | |
| ND = Not determined. | |||||||||
| 0.5 g IntravenousIntravenous doses were infused at a constant rate over 30 minutes. | 82 | 59 | 48 | 37 | 29 | 23 | 15 | 10 | 5 |
| 0.5 g Intramuscular 250 mg/mL | 22 | 33 | 38 | 35 | 30 | 26 | 16 | ND | 5 |
| 0.5 g Intramuscular 350 mg/mL | 20 | 32 | 38 | 34 | 31 | 24 | 16 | ND | 5 |
| 1 g Intravenous | 151 | 111 | 88 | 67 | 53 | 43 | 28 | 18 | 9 |
| 1 g Intramuscular | 40 | 68 | 76 | 68 | 56 | 44 | 29 | ND | ND |
| 2 g Intravenous | 257 | 192 | 154 | 117 | 89 | 74 | 46 | 31 | 15 |
Ceftriaxone was completely absorbed following intramuscular administration with mean maximum plasma concentrations occurring between 2 and 3 hours post-dose. Multiple intravenous or intramuscular doses ranging from 0.5 to 2 g at 12- to 24-hour intervals resulted in 15% to 36% accumulation of ceftriaxone above single dose values.
Ceftriaxone concentrations in urine are shown in Table 2.
| Dose/Route | Average Urinary Concentrations (mcg/mL) | |||||
|---|---|---|---|---|---|---|
| 0 to 2 hr | 2 to 4 hr | 4 to 8 hr | 8 to 12 hr | 12 to 24 hr | 24 to 48 hr | |
| ND = Not determined. | ||||||
| 0.5 g Intravenous | 526 | 366 | 142 | 87 | 70 | 15 |
| 0.5 g Intramuscular | 115 | 425 | 308 | 127 | 96 | 28 |
| 1 g Intravenous | 995 | 855 | 293 | 147 | 132 | 32 |
| 1 g Intramuscular | 504 | 628 | 418 | 237 | ND | ND |
| 2 g Intravenous | 2692 | 1976 | 757 | 274 | 198 | 40 |
Thirty-three percent to 67% of a ceftriaxone dose was excreted in the urine as unchanged drug and the remainder was secreted in the bile and ultimately found in the feces as microbiologically inactive compounds. After a 1 g intravenous dose, average concentrations of ceftriaxone, determined from 1 to 3 hours after dosing, were 581 mcg/mL in the gallbladder bile, 788 mcg/mL in the common duct bile, 898 mcg/mL in the cystic duct bile, 78.2 mcg/g in the gallbladder wall and 62.1 mcg/mL in the concurrent plasma.
Over a 0.15 to 3 g dose range in healthy adult subjects, the values of elimination half-life ranged from 5.8 to 8.7 hours; apparent volume of distribution from 5.78 to 13.5 L; plasma clearance from 0.58 to 1.45 L/hour; and renal clearance from 0.32 to 0.73 L/hour. Ceftriaxone is reversibly bound to human plasma proteins, and the binding decreased from a value of 95% bound at plasma concentrations of < 25 mcg/mL to a value of 85% bound at 300 mcg/mL. Ceftriaxone crosses the blood placenta barrier.
The average values of maximum plasma concentration, elimination half-life, plasma clearance and volume of distribution after a 50 mg/kg intravenous dose and after a 75 mg/kg intravenous dose in pediatric patients suffering from bacterial meningitis are shown in Table 3. Ceftriaxone penetrated the inflamed meninges of infants and pediatric patients; CSF concentrations after a 50 mg/kg intravenous dose and after a 75 mg/kg intravenous dose are also shown in Table 3.
| 50 mg/kg Intravenous | 75 mg/kg Intravenous | |
|---|---|---|
| Maximum Plasma Concentrations (mcg/mL) | 216 | 275 |
| Elimination Half-life (hr) | 4.6 | 4.3 |
| Plasma Clearance (mL/hr/kg) | 49 | 60 |
| Volume of Distribution (mL/kg) | 338 | 373 |
| CSF Concentration - inflamed meninges (mcg/mL) | 5.6 | 6.4 |
| Range (mcg/mL) | 1.3 to 18.5 | 1.3 to 44 |
| Time after dose (hr) | 3.7 (± 1.6) | 3.3 (± 1.4) |
Compared to that in healthy adult subjects, the pharmacokinetics of ceftriaxone were only minimally altered in elderly subjects and in patients with renal impairment or hepatic dysfunction (Table 4); therefore, dosage adjustments are not necessary for these patients with ceftriaxone dosages up to 2 g per day. Ceftriaxone was not removed to any significant extent from the plasma by hemodialysis. In 6 of 26 dialysis patients, the elimination rate of ceftriaxone was markedly reduced.
| Subject Group | Elimination Half-Life (hr) | Plasma Clearance (L/hr) | Volume of Distribution (L) |
|---|---|---|---|
| Healthy Subjects | 5.8 to 8.7 | 0.58 to 1.45 | 5.8 to 13.5 |
| Elderly Subjects (mean age, 70.5 yr) | 8.9 | 0.83 | 10.7 |
| Patients With Renal Impairment | |||
| Hemodialysis Patients (0 to 5 mL/min)Creatinine clearance. | 14.7 | 0.65 | 13.7 |
| Severe (5 to 15 mL/min) | 15.7 | 0.56 | 12.5 |
| Moderate (16 to 30 mL/min) | 11.4 | 0.72 | 11.8 |
| Mild (31 to 60 mL/min) | 12.4 | 0.70 | 13.3 |
| Patients With Liver Disease | 8.8 | 1.1 | 13.6 |
The elimination of ceftriaxone is not altered when ceftriaxone is co-administered with probenecid.