Ceretec
(Technetium Tc-99m Exametazime)Ceretec Prescribing Information
Technetium Tc99m exametazime scintigraphy may be useful as an adjunct in the detection of altered regional cerebral perfusion in stroke.
Tc99m exametazime is indicated for leukocyte labeled scintigraphy as an adjunct in the localization of intra-abdominal infection and inflammatory bowel disease.
The normal adult (70 kg) dose is 0.259-0.925 GBq (7-25 mCi) as Tc99m labeled leukocytes by intravenous injection. Optimal planar imaging is between 2-4 hours.
None known.
Rash with generalized erythema, facial edema and fever has been reported in less than 1% of patients. A transient increase in blood pressure was seen in 8% of patients.
The Ceretec kit is supplied as a pack of 5 vials for use in the preparation of a technetium Tc99m exametazime intravenous injection as a diagnostic radiopharmaceutical for use as an adjunct in the detection of altered regional cerebral perfusion and for the radiolabeling of autologous leukocytes. Each vial of Ceretec contains a pre-dispensed sterile, non-pyrogenic, lyophilized mixture of 0.5 mg exametazime [(RR,SS)-4.8-diaza-3,6,6,9-tetramethylundecane-2, 10-dione bisoxime], 7.6 µg stannous chloride dihydrate (minimum stannous tin 0.6 µg; maximum total stannous and stannic tin 4.0 µg per vial) and 4.5 mg sodium chloride, sealed under nitrogen atmosphere with a rubber closure. The product contains no antimicrobial preservative.
Prior to publication of the USAN, exametazime was formerly known as hexamethylpropylene amine oxime (HM-PAO). The name HM-PAO appears in many publications.
The structural formula of exametazime is:
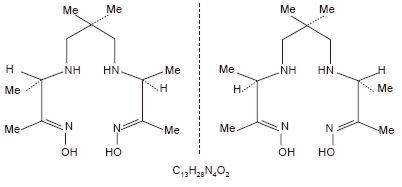
When sterile pyrogen-free sodium pertechnetate Tc99m in isotonic saline is added to the vial of Ceretec, a Tc99m complex of exametazime is formed.
Administration is by intravenous injection for diagnostic use.
When technetium Tc99m pertechnetate is added to exametazime in the presence of stannous reductant, a lipophilic technetium Tc99m complex is formed. This lipophilic complex is the active moiety. It converts at approximately 12%/hour to less lipophilic species. When the secondary complex is separated from the lipophilic species, it is unable to cross the blood-brain-barrier. The useful life of the reconstituted agent is limited to 30 minutes.