Cetrorelix Acetate
Cetrorelix Acetate Prescribing Information
Cetrorelix Acetate for Injection is indicated for the inhibition of premature LH surges in women undergoing controlled ovarian stimulation.
Ovarian stimulation therapy with gonadotropins (FSH, hMG) is started on cycle Day 2 or 3. The dose of gonadotropins should be adjusted according to individual response. Cetrorelix acetate for injection 0.25 mg may be administered subcutaneously once daily during the early- to mid-follicular phase.
When assessment by ultrasound shows a sufficient number of follicles of adequate size, hCG is administered to induce ovulation and final maturation of the oocytes. No hCG should be administered if the ovaries show an excessive response to the treatment with gonadotropins to reduce the chance of developing ovarian hyperstimulation syndrome (OHSS).
Cetrorelix acetate is contraindicated under the following conditions:
- Hypersensitivity to cetrorelix acetate, extrinsic peptide hormones or mannitol.
- Known hypersensitivity to GnRH or any other GnRH analogs.
- Known or suspected pregnancy, and lactation (see ).
PRECAUTIONSGeneralCases of hypersensitivity reactions, including anaphylactoid reactions with the first dose, have been reported during post-marketing surveillance (see
ADVERSE REACTIONS). A severe anaphylactic reaction associated with cough, rash, and hypotension, was observed in one patient after seven months of treatment with cetrorelix acetate (10 mg/day) in a study for an indication unrelated to infertility.Special care should be taken in women with signs and symptoms of active allergic conditions or known history of allergic predisposition. Treatment with cetrorelix acetate is not advised in women with severe allergic conditions.
Information for PatientsPrior to therapy with cetrorelix acetate, patients should be informed of the duration of treatment and monitoring procedures that will be required. The risk of possible adverse reactions should be discussed (see
ADVERSE REACTIONS).Cetrorelix acetate should not be prescribed if a patient is pregnant.If cetrorelix acetate is prescribed to patients for self-administration, information for proper use is given in the Patient Leaflet (see below).
Laboratory TestsAfter the exclusion of preexisting conditions, enzyme elevations (ALT, AST, GGT, alkaline phosphatase) were found in 1-2% of patients receiving cetrorelix acetate during controlled ovarian stimulation. The elevations ranged up to three times the upper limit of normal. The clinical significance of these findings was not determined.
During stimulation with human menopausal gonadotropin, cetrorelix acetate had no notable effects on hormone levels aside from inhibition of LH surges.
Drug InteractionsNo formal drug interaction studies have been performed with cetrorelix acetate.
Carcinogenesis, Mutagenesis, Impairment of FertilityLong-term carcinogenicity studies in animals have not been performed with cetrorelix acetate. Cetrorelix acetate was not genotoxic
in vitro(Ames test, HPRT test, chromosome aberration test) orin vivo(chromosome aberration test, mouse micronucleus test). Cetrorelix acetate induced polyploidy in CHL-Chinese hamster lung fibroblasts, but not in V79-Chinese hamster lung fibroblasts, cultured peripheral human lymphocytes or in anin vitromicronucleus test in the CHL-cell line. Treatment with 0.46 mg/kg cetrorelix acetate for 4 weeks resulted in complete infertility in female rats which was reversed 8 weeks after cessation of treatment.Pregnancy (see CONTRAINDICATIONS)Cetrorelix acetate is contraindicated in pregnant women.
When administered to rats for the first seven days of pregnancy, cetrorelix acetate did not affect the development of the implanted conceptus at doses up to 38 mcg/kg (approximately 1 times the recommended human therapeutic dose based on body surface area). However, a dose of 139 mcg/kg (approximately 4 times the human dose) resulted in a resorption rate and a post-implantation loss of 100%.
When administered from day 6 to near term to pregnant rats and rabbits, very early resorptions and total implantation losses were seen in rats at doses from 4.6 mcg/kg (0.2 times the human dose) and in rabbits at doses from 6.8 mcg/kg (0.4 times the human dose). In animals that maintained their pregnancy, there was no increase in the incidence of fetal abnormalities.The fetal resorption observed in animals is a logical consequence of the alteration in hormonal levels effected by the antigonadotrophic properties of cetrorelix acetate, which could result in fetal loss in humans as well. Therefore, this drug should not be used in pregnant women.
Nursing MothersIt is not known whether cetrorelix acetate is excreted in human milk. Because many drugs are excreted in human milk, and because the effects of cetrorelix acetate on lactation and/or the breast-fed child have not been determined, cetrorelix acetate should not be used by nursing mothers.
Geriatric UseCetrorelix acetate is not intended to be used in subjects aged 65 and over.
- Severe renal impairment
The safety of cetrorelix acetate in 949 patients undergoing controlled ovarian stimulation in clinical studies was evaluated. Women were between 19 and 40 years of age (mean: 32). 94.0% of them were Caucasian. Cetrorelix acetate was given in doses ranging from 0.1 mg to 5 mg as either a single or multiple dose.
* Intensity moderate or severe, or WHO Grade II or III, respectively | |
(WHO preferred term) | Cetrorelix Acetate N=949 % (n) |
| Ovarian Hyperstimulation Syndrome* | 3.5 (33) |
| Nausea | 1.3 (12) |
| Headache | 1.1 (10) |
* Intensity moderate or severe, or WHO Grade II or III, respectively | |
(WHO preferred term) | Cetrorelix Acetate N=949 % (n) |
| Ovarian Hyperstimulation Syndrome* | 3.5 (33) |
| Nausea | 1.3 (12) |
| Headache | 1.1 (10) |
Local site reactions (e.g. redness, erythema, bruising, itching, swelling, and pruritus) were reported. Usually, they were of a transient nature, mild intensity and short duration. During post-marketing surveillance, cases of mild to moderate Ovarian Hyperstimulation syndrome and cases of hypersensitivity reactions including anaphylactoid reactions have been reported.
Two stillbirths were reported in Phase 3 studies of cetrorelix acetate.
No formal drug interaction studies have been performed with cetrorelix acetate.
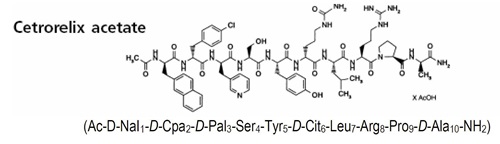
Cetrorelix Acetate for Injection is a synthetic decapeptide with gonadotropin-releasing hormone (GnRH) antagonistic activity. Cetrorelix acetate is an analog of native GnRH with substitutions of amino acids at positions 1, 2, 3, 6, and 10. The molecular formula is Acetyl-D-3-(2′-naphtyl)-alanine-D-4-chlorophenylalanine-D-3-(3′-pyridyl)-alanine-L-serine-L-tyrosine-D-citruline-L-leucine-L-arginine-L-proline-D-alanine-amide, and the molecular weight is 1431.06, calculated as the anhydrous free base. The structural formula is as follows:
Cetrorelix Acetate for Injection 0.25 mg is a sterile lyophilized powder intended for subcutaneous injection after reconstitution with Sterile Water for Injection, that comes supplied in a 1 mL prefilled syringe. Each vial of Cetrorelix Acetate for Injection 0.25 mg contains 0.26-0.27 mg cetrorelix acetate, equivalent to 0.25 mg cetrorelix, and 54.80 mg mannitol.