Chloramphenicol Sodium Succinate Prescribing Information
It is not recommended for the routine treatment of the typhoid carrier state.
a)
b)
c)
d) Lymphogranuloma-psittacosis group
e) Various gram-negative bacteria causing bacteremia, meningitis or other serious gram-negative infections.
f) Other susceptible organisms which have been demonstrated to be resistant to all other appropriate antimicrobial agents.
*In treatment of typhoid fever some authorities recommend that chloramphenicol be administered at therapeutic levels for 8 to 10 days after the patient has become afebrile to lessen the possibility of relapse.
Chloramphenicol is contraindicated in individuals with a history of previous hypersensitivity and/or toxic reaction to it.
Toxic reactions including fatalities have occurred in the premature and neonate; the signs and symptoms associated with these reactions have been referred to as the “gray syndrome.” One case of gray syndrome has been reported in a neonate born to a mother having received chloramphenicol during labor. One case has been reported in a 3-month-old infant. The following summarizes the clinical and laboratory studies that have been made on these patients:
a) In most cases therapy with chloramphenicol had been instituted within the first 48 hours of life.
b) Symptoms first appeared after 3 to 4 days of continued treatment with high doses of chloramphenicol.
c) The symptoms appeared in the following order:
(1) abdominal distension with or without emesis;
(2) progressive pallid cyanosis;
(3) vasomotor collapse, frequently accompanied by irregular respiration;
(4) death within a few hours of onset of these symptoms.
d) The progression of symptoms from onset to exitus was accelerated with higher dose schedules.
e) Preliminary blood serum level studies revealed unusually high concentrations of chloramphenicol (over 90 mcg/mL after repeated doses).
f) Termination of therapy upon early evidence of the associated symptomatology frequently reversed the process with complete recovery.
Concurrent therapy with other drugs that may cause bone marrow depression should be avoided.
Chloramphenicol is an antibiotic that is clinically useful for,
It is not recommended for the routine treatment of the typhoid carrier state.
a)
b)
c)
d) Lymphogranuloma-psittacosis group
e) Various gram-negative bacteria causing bacteremia, meningitis or other serious gram-negative infections.
f) Other susceptible organisms which have been demonstrated to be resistant to all other appropriate antimicrobial agents.
*In treatment of typhoid fever some authorities recommend that chloramphenicol be administered at therapeutic levels for 8 to 10 days after the patient has become afebrile to lessen the possibility of relapse.
When reconstituted as directed, each vial contains a sterile solution equivalent to 100 mg of chloramphenicol per mL (1 g/10 mL).
Each gram (10 mL of a 10% solution) of chloramphenicol sodium succinate contains approximately 52 mg (2.25 mEq) of sodium.
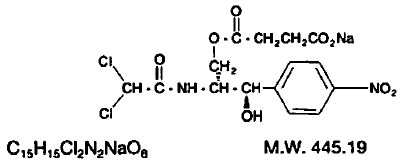
The chemical name for chloramphenicol sodium succinate is D-threo-(-)-2, 2-Dichloro-N-[β-hydroxy-α-(hydroxymethyl)-p-nitrophenethyl] acetamide α-(sodium succinate).
The structural formula is:
When available, the clinical microbiology laboratory should provide cumulative reports of
Quantitative methods are used to determine antimicrobial minimum inhibitory concentrations (MICs). These MICs provide estimates of the susceptibility of bacteria to antimicrobial compounds. The MICs should be determined using a standardized (broth and/or agar).
1,3 The MIC values should be interpreted according to the criteria provided in Table 1.
Quantitative methods that require measurement of zone diameters can also provide reproducible estimates of the susceptibility of bacteria to antimicrobial compounds. The zone size should be determined using a standardized test method.
2,3 This procedure uses paper disks impregnated with 30 mcg chloramphenicol to test the susceptibility of bacteria to chloramphenicol. The disc diffusion breakpoints should be interpreted according to the criteria provided in Table 1.
Table 1. Susceptibility Test Interpretive Criteria for Chloramphenicol | ||||||
Pathogen | Minimum Inhibitory Concentrations (mcg/mL) | Zone Diameters (mm) | ||||
S | I | R | S | I | R | |
Salmonella spp. | < 8 | 16 | > 32 | > 18 | 13 to 17 | < 12 |
Haemophilus influenzae | < 2 | 4 | > 8 | ≥ 29 | 26 to 28 | < 25 |
A report of
Quality Control
Standardized susceptibility test procedures require the use of laboratory controls to monitor and ensure the accuracy and precision of supplies and reagents used in the assay, and the techniques of the individuals performing the test.
1,2,3 Standard chloramphenicol powder should provide the following range of MIC values noted in Table 2. For the disc diffusion technique using the 30 mcg disk, the criteria in Table 2 should be achieved.
Table 2. Quality Control Parameters for Chloramphenicol | |||||
QC Strain | Minimum Inhibitory Concentrations (mcg/mL) | Zone Diameters (mm) | |||
Escherichia coli ATCC 25922 | 2 to 8 | 21 to 27 | |||
Haemophilus influenzae ATCC 49247 | 0.25 to 1 | 31 to 40 | |||