Chlordiazepoxide Hydrochloride And Clidinium Bromide - Chlordiazepoxide Hydrochloride And Clidinium Bromide capsule prescribing information
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (see WARNINGS and PRECAUTIONS and PRECAUTIONS, Drug Interactions).
- The use of benzodiazepines, including chlordiazepoxide hydrochloride, a component of chlordiazepoxide hydrochloride and clidinium bromide capsules, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing chlordiazepoxide hydrochloride and clidinium bromide capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see WARNINGS) .
- The continued use of benzodiazepines, including chlordiazepoxide hydrochloride and clidinium bromide capsules, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide hydrochloride and clidinium bromide capsules after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide hydrochloride and clidinium bromide capsules or reduce the dosage (see WARNINGS and DOSAGE AND ADMINISTRATION).
INDICATIONS AND USAGE
Chlordiazepoxide hydrochloride and clidinium bromide capsules are indicated to control emotional and somatic factors in gastrointestinal disorders. Chlordiazepoxide hydrochloride and clidinium bromide capsules may also be used as adjunctive therapy in the treatment of peptic ulcer and in the treatment of the irritable bowel syndrome (irritable colon, spastic colon, mucous colitis) and acute enterocolitis.
DOSAGE AND ADMINISTRATION
Recommended Dosage
Because of the varied individual responses to tranquilizers and anticholinergics, the optimum dosage of chlordiazepoxide hydrochloride and clidinium bromide capsules varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects. The usual maintenance dose is 1 or 2 capsules, 3 or 4 times a day administered before meals and at bedtime.
Recommended Geriatric Dosage
Dosage should be limited to the smallest effective amount to preclude the development of ataxia, oversedation or confusion. The initial dose should not exceed 2 chlordiazepoxide hydrochloride and clidinium bromide capsules per day, to be increased gradually as needed and tolerated. Elderly patients have an increased risk of dose-related adverse reactions (see PRECAUTIONS ).
Discontinuation or Dosage Reduction of Chlordiazepoxide Hydrochloride and Clidinium Bromide Capsules
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide hydrochloride and clidinium bromide capsules or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS and DRUG ABUSE AND DEPENDENCE ).
CONTRAINDICATIONS
Chlordiazepoxide hydrochloride and clidinium bromide capsules are contraindicated in the presence of glaucoma (since the anticholinergic component may produce some degree of mydriasis) and in patients with prostatic hypertrophy and benign bladder neck obstruction. It is contraindicated in patients with known hypersensitivity to chlordiazepoxide hydrochloride and/or clidinium bromide.
ADVERSE REACTIONS
No side effects or manifestations not seen with either compound alone have been reported with the administration of chlordiazepoxide hydrochloride and clidinium bromide capsules. However, since chlordiazepoxide hydrochloride and clidinium bromide capsules contains chlordiazepoxide hydrochloride and clidinium bromide, the possibility of untoward effects which may be seen with either of these two compounds cannot be excluded.
When chlordiazepoxide hydrochloride has been used alone the necessity of discontinuing therapy because of undesirable effects has been rare. Drowsiness, ataxia and confusion have been reported in some patients — particularly the elderly and debilitated. While these effects can be avoided in almost all instances by proper dosage adjustment, they have occasionally been observed at the lower dosage ranges. In a few instances syncope has been reported.
Other adverse reactions reported during therapy with chlordiazepoxide hydrochloride include isolated instances of skin eruptions, edema, minor menstrual irregularities, nausea and constipation, extrapyramidal symptoms, as well as increased and decreased libido. Such side effects have been infrequent and are generally controlled with reduction of dosage. Changes in EEG patterns (low-voltage fast activity) have been observed in patients during and after chlordiazepoxide hydrochloride treatment.
Blood dyscrasias, including agranulocytosis, jaundice and hepatic dysfunction have occasionally been reported during therapy with chlordiazepoxide hydrochloride. When chlordiazepoxide hydrochloride treatment is protracted, periodic blood counts and liver function tests are advisable.
Adverse effects reported with use of chlordiazepoxide hydrochloride and clidinium bromide capsules are those typical of anticholinergic agents, i.e., dryness of the mouth, blurring of vision, urinary hesitancy and constipation. Constipation has occurred most often when chlordiazepoxide hydrochloride and clidinium bromide therapy has been combined with other spasmolytic agents and/or a low residue diet.
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Opioids
The concomitant use of benzodiazepines, including chlordiazepoxide hydrochloride, a component of chlordiazepoxide hydrochloride and clidinium bromide capsules and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABA A sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of chlordiazepoxide hydrochloride and clidinium bromide capsules and opioids, and follow patients closely for respiratory depression and sedation.
Oral Anticoagulants
Although clinical studies have not established a cause and effect relationship, physicians should be aware that variable effects on blood coagulation have been reported very rarely in patients receiving oral anticoagulants and chlordiazepoxide hydrochloride, a component of chlordiazepoxide hydrochloride and clidinium bromide capsules.
DESCRIPTION
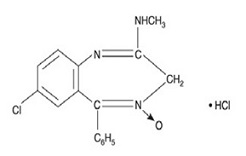
Chlordiazepoxide hydrochloride and clidinium bromide capsules, USP are a fixed-combination of chlordiazepoxide hydrochloride, a benzodiazepine, and clidinium bromide, an anticholinergic. Each chlordiazepoxide hydrochloride and clidinium bromide capsule, USP contains the active ingredients 5 mg chlordiazepoxide hydrochloride, USP and 2.5 mg clidinium bromide, USP. Each capsule also contains the inactive ingredients lactose monohydrate, corn starch, talc, titanium dioxide, FD&C Green 3, D&C Yellow 10 and gelatin. The empty hard gelatin capsule shells are printed with edible black ink containing shellac, propylene glycol, black iron oxide and potassium hydroxide. Chlordiazepoxide hydrochloride, USP is 7-chloro-2-methylamino-5-phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride. A white or practically white crystalline powder, it is soluble in water, sparingly soluble in alcohol and insoluble in solvent hexane. It is unstable in solution and the powder must be protected from light. The molecular weight is 336.22. The structural formula of chlordiazepoxide hydrochloride, USP is as follows:
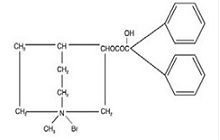
Clidinium bromide, USP is a synthetic anticholinergic agent which has been shown in experimental and clinical studies to have antispasmodic and antisecretory effects on the gastrointestinal tract.
Structurally clidinium bromide, USP is:
Product meets USP Dissolution Test 2.
ANIMAL PHARMACOLOGY AND/OR ANIMAL TOXICOLOGY
Effects on Reproduction
Reproduction studies in rats fed chlordiazepoxide hydrochloride, 10, 20 and 80 mg/kg daily (2.4, 4.8 and 19.4 times, respectively, the maximum recommended clinical dose of 40 mg/day, based on body surface area), and bred through one or two matings showed no congenital anomalies, nor were there adverse effects on growth of the newborn. However, in another study at 100 mg/kg daily there was noted a significant decrease in the fertilization rate and a marked decrease in the viability and body weight of offspring which may be attributable to sedative activity, thus resulting in lack of interest in mating and lessened maternal nursing and care of the young. One neonate in each of the first and second matings in the rat reproduction study at the 100 mg/kg dose (24.2 times the maximum recommended human dose of 40 mg/day, based on body surface area) exhibited major skeletal defects.
Two series of reproduction experiments with clidinium bromide were carried out in rats, employing dosages of 2.5 and 10 mg/kg daily (1.2 and 4.9 times, respectively, the maximum recommended clinical dose of 20 mg/day, based on body surface area) in each experiment. In the first experiment, clidinium bromide was administered for a 9-week interval prior to mating; no untoward effect on fertilization or gestation was noted. The offspring were taken by caesarean section and did not show a significant incidence of congenital anomalies when compared to control animals. In the second experiment, adult animals were given clidinium bromide for 10 days prior to and through two mating cycles. No significant effects were observed on fertility, gestation, viability of offspring or lactation, as compared to control animals, nor was there a significant incidence of congenital anomalies in the offspring derived from these experiments.
A reproduction study was carried out in rats through two successive matings with administration of oral daily doses of 2.5 mg/kg chlordiazepoxide hydrochloride and 1.25 mg/kg clidinium bromide (0.6 times the maximum recommended clinical dose for both drugs, based on body surface area) or 25 mg/kg chlordiazepoxide hydrochloride and 12.5 mg/kg clidinium bromide (6.1 times the maximum recommended clinical dose for both drugs, based on body surface area). In the first mating, no significant differences were noted between the control or the treated groups, with the exception of a slight decrease in the number of animals surviving during lactation among those receiving the high dosage. In the second mating, similar results were obtained except for a slight decrease in the number of pregnant females and in the percentage of offspring surviving until weaning. No congenital anomalies were observed in both matings in either the control or treated groups.
HOW SUPPLIED
Chlordiazepoxide hydrochloride and clidinium bromide capsules, USP are opaque light green cap/opaque light green body hard gelatin capsule, size “3” having imprinting “A” on cap and “333” on body with black ink, filled with white to off white powder.
Bottle of 100 capsules with child resistant closure, NDC 46708-744-31 Bottle of 1000 capsules, NDC 46708-744-91
Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Protect from light.
Keep out of reach of children.
Dispense in tight, light-resistant container as defined in USP/NF.
Manufactured by: Alembic Pharmaceuticals Limited (Formulation Division), Village Panelav, P. O. Tajpura, Near Baska, Taluka-Halol, Panchmahal 389350, Gujarat, India.
Revised: 02/2023