Chlordiazepoxide Hydrochloride Prescribing Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation (seeAND
WARNINGSRisks from Concomitant Use with Opioids:Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction:The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions:To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Neonatal Sedation and Withdrawal Syndrome
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (seePRECAUTIONS, Pregnancy). Monitor neonates exposed to Chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation and monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal; manage these infants accordingly.).PRECAUTIONSIn elderly and debilitated patients, it is recommended that the dosage be limited to the smallest effective amount to preclude the development of ataxia or oversedation (10 mg or less per day initially, to be increased gradually as needed and tolerated). In general, the concomitant administration of chlordiazepoxide and other psychotropic agents is not recommended. If such combination therapy seems indicated, careful consideration should be given to the pharmacology of the agents to be employed particularly when the known potentiating compounds such as MAO inhibitors and phenothiazines are to be used. The usual precautions in treating patients with impaired renal or hepatic function should be observed.
Paradoxical reactions, e.g., excitement, stimulation and acute rage, have been reported in psychiatric patients and in hyperactive aggressive pediatric patients, and should be watched for during chlordiazepoxide therapy. The usual precautions are indicated when chlordiazepoxide is used in the treatment of anxiety states where there is any evidence of impending depression; it should be borne in mind that suicidal tendencies may be present and protective measures may be necessary. Although clinical studies have not established a cause and effect relationship, physicians should be aware that variable effects on blood coagulation have been reported very rarely in patients receiving oral anticoagulants and chlordiazepoxide. In view of isolated reports associating chlordiazepoxide with exacerbation of porphyria, caution should be exercised in prescribing chlordiazepoxide to patients suffering from this disease.
Pediatric UseBecause of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required (see DOSAGE AND ADMINISTRATION). Since clinical experience with chlordiazepoxide in pediatric patients under 6 years of age is limited, use in this age group is not recommended. Hyperactive aggressive pediatric patients should be monitored for paradoxical reactions to chlordiazepoxide (see PRECAUTIONS).
Information for PatientsAdvise the patient to read the FDA-approved patient labeling .
Risks from Concomitant Use with OpioidsAdvise both patients and caregivers about the risk of potentially fatal respiratory depression and sedation when chlordiazepoxide is used with opioids and not to use such drugs concomitantly unless supervised by a healthcare provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS: Risks from Concomitant Use with Opioidsand PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and AddictionInform patients that the use of chlordiazepoxide, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS: Abuse, Misuse, and Addictionand DRUG ABUSE AND DEPENDENCE).
Withdrawal ReactionsInform patients that the continued use of chlordiazepoxide may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of chlordiazepoxide may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of chlordiazepoxide may require a slow taper (see WARNINGS: Dependence and Withdrawal Reactionsand DRUG ABUSE AND DEPENDENCE).
Pregnancy
Advise pregnant females that use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns(seeWarnings, Neonatal Sedationand Withdrawal Syndromeand Precautions, Pregnancy).
Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to chlordiazepoxide hydrochloride during pregnancy (seePrecautions, Pregnancy).Nursing
Advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide hydrochloride (see Precautions, Nursing Mothers).Drug InteractionsThe concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists.
Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
Pregnancy:
Pregnancy Exposure Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including chlordiazepoxide hydrochloride, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (seeWARNINGS, Neonatal Sedation and Withdrawal Syndrome,and Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical ConsiderationsFetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation, respiratory depression, hypotonia, and feeding problems Monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal. Manage these neonates accordingly (seeWARNINGS, Neonatal Sedationand Withdrawal Syndrome).
DataHuman Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.:Nursing
Risk Summary
There are no data on the presence of chlordiazepoxide in human milk. There are reports of sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. Because of chlordiazepoxide’s long half-life, the potential for chlordiazepoxide to accumulate in breast milk and the potential for serious adverse reactions, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with chlordiazepoxide hydrochloride. - The use of benzodiazepines, including chlordiazepoxide hydrochloride capsules, exposes users to risk of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing chlordiazepoxide hydrochloride capsules and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (see).
WARNINGSRisks from Concomitant Use with Opioids:Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction:The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions:To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Neonatal Sedation and Withdrawal Syndrome
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (seePRECAUTIONS, Pregnancy). Monitor neonates exposed to Chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation and monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal; manage these infants accordingly. - The continued use of benzodiazepines, including chlordiazepoxide hydrochloride capsules, may lead to clinically significant physical dependence. The risk of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide hydrochloride capsules after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide hydrochloride capsules or reduce the dosage (seeand
DOSAGE AND ADMINISTRATIONBecause of the wide range of clinical indications for chlordiazepoxide, the optimum dosage varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects.
AdultsUsual Daily DoseRelief of Mild and Moderate Anxiety Disorders and Symptoms of Anxiety5 mg or 10 mg, 3 or 4 times daily Relief of Severe Anxiety Disorders and Symptoms of Anxiety20 mg or 25 mg, 3 or 4 times daily
Geriatric Patients, or in the presence of debilitating disease5 mg, 2 to 4 times daily
Preoperative Apprehension and Anxiety: On days preceding surgery, 5 to 10 mg orally, 3 or 4 times daily. If used as preoperative medication, 50 to 100 mg IM* 1 hour prior to surgeryPEDIATRIC PATIENTS USUAL DAILY DOSE Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required.
Since clinical experience in pediatric patients under 6 years of age is limited, the use of the drug in this age group is not recommended.5 mg, 2 to 4 times daily (may be increased in some pediatric patients to 10 mg, 2 to 3 times daily) For the relief of withdrawal symptoms of acute alcoholism, the parenteral form* is usually used initially. If the drug is administered orally, the suggested initial dose is 50 to 100 mg, to be followed by repeated doses as needed until agitation is controlled - up to 300 mg per day. Dosage should then be reduced to maintenance levels.
*See package insert for Injectable Chlordiazepoxide HydrochlorideDiscontinuation or Dosage Reduction of Chlordiazepoxide
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increase the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS: Dependence and Withdrawal Reactionsand DRUG ABUSE AND DEPENDENCE: Dependence).).WARNINGSRisks from Concomitant Use with Opioids:Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
Abuse, Misuse, and Addiction:The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions:To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
Acute Withdrawal ReactionsThe continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
Protracted Withdrawal SyndromeIn some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Neonatal Sedation and Withdrawal Syndrome
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (seePRECAUTIONS, Pregnancy). Monitor neonates exposed to Chlordiazepoxide hydrochloride during pregnancy or labor for signs of sedation and monitor neonates exposed to chlordiazepoxide hydrochloride during pregnancy for signs of withdrawal; manage these infants accordingly.
Chlordiazepoxide Hydrochloride Capsules are indicated for the management of anxiety disorders or for the short term relief of symptoms of anxiety, withdrawal symptoms of acute alcoholism, and preoperative apprehension and anxiety. Anxiety or tension associated with the stress of everyday life usually does not require treatment with an anxiolytic.
The effectiveness of chlordiazepoxide hydrochloride capsules in long-term use, that is, more than 4 months, has not been assessed by systematic clinical studies. The physician should periodically reassess the usefulness of the drug for the individual patient.
Because of the wide range of clinical indications for chlordiazepoxide, the optimum dosage varies with the diagnosis and response of the individual patient. The dosage, therefore, should be individualized for maximum beneficial effects.
Adults | Usual Daily Dose |
Relief of Mild and Moderate Anxiety Disorders and Symptoms of Anxiety | 5 mg or 10 mg, 3 or 4 times daily |
Relief of Severe Anxiety Disorders and Symptoms of Anxiety | 20 mg or 25 mg, 3 or 4 times daily |
Geriatric Patients, or in the presence of debilitating disease | 5 mg, 2 to 4 times daily |
Preoperative Apprehension and Anxiety: On days preceding surgery, 5 to 10 mg orally, 3 or 4 times daily. If used as preoperative medication, 50 to 100 mg IM* 1 hour prior to surgery | |
| PEDIATRIC PATIENTS | USUAL DAILY DOSE |
Because of the varied response of pediatric patients to CNS-acting drugs, therapy should be initiated with the lowest dose and increased as required. Since clinical experience in pediatric patients under 6 years of age is limited, the use of the drug in this age group is not recommended. | 5 mg, 2 to 4 times daily (may be increased in some pediatric patients to 10 mg, 2 to 3 times daily) |
For the relief of withdrawal symptoms of acute alcoholism, the parenteral form* is usually used initially. If the drug is administered orally, the suggested initial dose is 50 to 100 mg, to be followed by repeated doses as needed until agitation is controlled - up to 300 mg per day. Dosage should then be reduced to maintenance levels.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increase the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see
Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
The continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (
Concomitant use of benzodiazepines, including chlordiazepoxide, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe chlordiazepoxide concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of chlordiazepoxide than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking chlordiazepoxide, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when chlordiazepoxide is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS: Drug Interactions).
The use of benzodiazepines, including chlordiazepoxide, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE: Abuse).
Before prescribing chlordiazepoxide and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of chlordiazepoxide, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of chlordiazepoxide along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue chlordiazepoxide or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION: Discontinuation or Dosage Reduction of Chlordiazepoxide).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages and those who have had longer durations of use.
The continued use of benzodiazepines, including chlordiazepoxide, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of chlordiazepoxide after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE: Dependence).
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE: Dependence).
Chlordiazepoxide Hydrochloride may impair the mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a vehicle or operating machinery. Similarly, it may impair mental alertness in children. The concomitant use of alcohol or other central nervous system depressants may have an additive effect. PATIENTS SHOULD BE WARNED ACCORDINGLY.
Use of chlordiazepoxide hydrochloride late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (
Chlordiazepoxide Hydrochloride Capsules are contraindicated in patients with known hypersensitivity to the drug.
The necessity of discontinuing therapy because of undesirable effects has been rare. Drowsiness, ataxia and confusion have been reported in some patients particularly the elderly and debilitated. While these effects can be avoided in almost all instances by proper dosage adjustment, they have occasionally been observed at the lower dosage ranges.
In a few instances syncope has been reported.
Other adverse reactions reported during therapy include isolated instances of skin eruptions, edema, minor menstrual irregularities, nausea and constipation, extrapyramidal symptoms, as well as increased and decreased libido. Such side effects have been infrequent, and are generally controlled with reduction of dosage. Changes in EEG patterns (low-voltage fast activity) have been observed in patients during and after chlordiazepoxide treatment.
Blood dyscrasias (including agranulocytosis), jaundice and hepatic dysfunction have occasionally been reported during therapy. When chlordiazepoxide treatment is protracted, periodic blood counts and liver function tests are advisable.
Chlordiazepoxide Hydrochloride, is the prototype for the benzodiazepine compounds. It is a versatile therapeutic agent of proven value for the relief of anxiety. Chlordiazepoxide Hydrochloride is among the safer of the effective psychopharmacologic compounds available, as demonstrated by extensive clinical evidence.
Chlordiazepoxide Hydrochloride is available as capsules containing 5 mg, 10 mg or 25 mg chlordiazepoxide hydrochloride. In addition, each capsule contains the following inactive ingredients:
5 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, black iron oxide, and propylene glycol.
10 mg: colloidal silicon dioxide, corn starch, D & C Yellow #10, FD & C Blue #1, FD & C Red #3, FD & C Yellow #6, gelatin, lactose monohydrate, talc, titanium dioxide, shellac, propylene glycol, and simethicone.
25 mg:
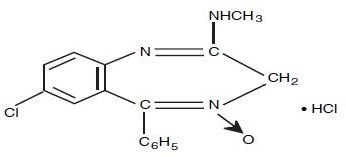
Chlordiazepoxide Hydrochloride is 7-chloro-2-(methylamino)-5-phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride. A white to practically white crystalline substance, it is soluble in water. It is unstable in solution and the powder must be protected from light. The molecular weight is 336.22. The structural formula of chlordiazepoxide hydrochloride is as follows: