Chloroquine Phosphate
Chloroquine Phosphate Prescribing Information
Chloroquine phosphate tablets are indicated for the:
- Treatment of uncomplicated malaria due to susceptible strains ofP. falciparum, P.malariae, P. ovale,andP.vivax.
- Prophylaxis of malaria in geographic areas where resistance to chloroquine is not present.
- Treatment of extraintestinal amebiasis.
Chloroquine phosphate tablets do not prevent relapses in patients with vivax or ovale malaria because it is not effective against exoerythrocytic forms of the parasites.
Limitations of Use in Malaria:
- Do not use Chloroquine phosphate tablets for the treatment of complicated malaria (high-grade parasitemia and/or complications e.g., cerebral malaria or acute renal failure).
- Do not use Chloroquine phosphate tablets for malaria prophylaxis in areas where chloroquine resistance occurs, Resistance to Chloroquine phosphate tablets is widespread inP. falciparum, and is reported inP.vivax(see WARNINGS).
- Concomitant therapy with an 8-aminoquinoline drug is necessary for treatment of the hypnozoite liver stage forms ofP.vivaxandP.ovale(see DOSAGE AND ADMINISTRATION).
The dosage of chloroquine phosphate is often expressed in terms of equivalent chloroquine base. Each 500 mg tablet of Chloroquine phosphate contains the equivalent of 300 mg chloroquine base. In infants and children the dosage is preferably calculated by body weight.
If circumstances permit, suppressive therapy should begin two weeks prior to exposure. However, failing this in adults, an initial double (loading) dose of 1 g (= 600 mg base), or in children 10 mg base/kg may be taken in two divided doses, six hours apart. The suppressive therapy should be continued for eight weeks after leaving the endemic area.
The dosage for adults of low body weight and for infants and children should be determined as follows:
First dose: 10 mg base per kg (but not exceeding a single dose of 600 mg base).
Second dose: (6 hours after first dose) 5 mg base per kg (but not exceeding a single dose of 300 mg base).
Third dose: (24 hours after first dose) 5 mg base per kg.
Fourth dose: (36 hours after first dose) 5 mg base per kg.
See PRECAUTIONS, Geriatric Use.
Use of Chloroquine phosphate tablets for indications other than acute malaria is contraindicated in the presence of retinal or visual field changes of any etiology.
Use of Chloroquine phosphate tablets is contraindicated in patients with known hypersensitivity to 4- aminoquinoline compounds.
The following adverse reactions have been identified during post-approval use of chloroquine or other 4-aminoqunoline compounds. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: Erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis. Pleomorphic skin eruptions, skin and mucosal pigmentary changes; lichen planus-like eruptions, pruritus,; drug rash with eosinophilia and systemic symptoms (DRESS syndrome); photosensitivity and hair loss and bleaching of hair pigment.
Cardiac arrhythmias, conduction disorders such as bundle branch block / atrio-ventricular block, QT interval prolongation, torsade de pointes, ventricular tachycardia and ventricular fibrillation have been reported with therapeutic doses of chloroquine as well as with overdose. The risk is greater if chloroquine is administered at high doses. Fatal cases have been reported (see WARNINGS, Cardiac Effects and OVERDOSAGE).
Chloroquine phosphate tablet, USP, is a 4-aminoquinoline compound for oral administration. It is a white, odorless, bitter tasting, crystalline substance, freely soluble in water.
Chloroquine phosphate tablet is an antimalarial and amebicidal drug.
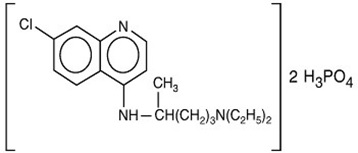
Chemically, it is 7-chloro-4-[[4-(diethylamino)-1-methylbutyl]amino]quinoline phosphate (1:2) and has the following structural formula:
Each tablet contains 500 mg of chloroquine phosphate USP, equivalent to 300 mg chloroquine base.
Chloroquine is rapidly and almost completely absorbed from the gastrointestinal tract, and only a small proportion of the administered dose is found in the stools. Approximately 55% of the drug in the plasma is bound to nondiffusible plasma constituents. Excretion of chloroquine is quite slow but is increased by acidification of the urine. Chloroquine is deposited in the tissues in considerable amounts. In animals, from 200 to 700 times the plasma concentration may be found in the liver, spleen, kidney, and lung; leukocytes also concentrate the drug. The brain and spinal cord, in contrast, contain only 10 to 30 times the amount present in plasma.
Chloroquine undergoes appreciable degradation in the body. The main metabolite is desethylchloroquine, which accounts for one fourth of the total material appearing in the urine; bisdesethylchloroquine, a carboxylic acid derivative, and other metabolic products as yet uncharacterized are found in small amounts. Slightly more than half of the urinary drug products can be accounted for as unchanged chloroquine.
QTc interval prolongation was studied in a randomized, placebo-controlled parallel trial in 116 healthy subjects who received either chloroquine (1000 mg) alone or in combination with oral azithromycin (500 mg, 1000 mg, and 1500 mg once daily). Co-administration of azithromycin increased the QTc interval in a dose- and concentration- dependent manner. In comparison to chloroquine alone, the maximum mean (95% upper confidence bound) increases in QTcF were 5 ms, 7 (12) ms and 9 (14) ms with the co-administration of 500 mg, 1000 mg and 1500 mg azithromycin, respectively.