Chlorpromazine Prescribing Information
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear.
Chlorpromazine hydrochloride is not approved for the treatment of patients with dementia-related psychosis
For the management of manifestations of psychotic disorders.
For the treatment of schizophrenia.
To control nausea and vomiting.
For relief of restlessness and apprehension before surgery.
For acute intermittent porphyria.
As an adjunct in the treatment of tetanus.
To control the manifestations of the manic type of manic-depressive illness.
For relief of intractable hiccups.
For the treatment of severe behavioral problems in children (1 to 12 years of age) marked by combativeness and/or explosive hyperexcitable behavior (out of proportion to immediate provocations), and in the short-term treatment of hyperactive children who show excessive motor activity with accompanying conduct disorders consisting of some or all of the following symptoms: impulsivity, difficulty sustaining attention, aggressivity, mood lability and poor frustration tolerance.
Adjust dosage to individual and the severity of his condition, recognizing that the milligram for milligram potency relationship among all dosage forms has not been precisely established clinically. It is important to increase dosage until symptoms are controlled. Dosage should be increased more gradually in debilitated or emaciated patients. In continued therapy, gradually reduce dosage to the lowest effective maintenance level, after symptoms have been controlled for a reasonable period.
The 100 mg and 200 mg tablets are for use in severe neuropsychiatric conditions.
Hospitalized Patients:
Chlorpromazine should generally not be used in pediatric patients under 6 months of age except where potentially lifesaving. It should not be used in conditions for which specific pediatric dosages have not been established.
Do not use in patients with known hypersensitivity to phenothiazines.
Do not use in comatose states or in the presence of large amounts of central nervous system depressants (alcohol, barbiturates, narcotics, etc.).
There is no conclusive evidence that preexisting liver disease makes patients more susceptible to jaundice. Alcoholics with cirrhosis have been successfully treated with chlorpromazine without complications. Nevertheless, the medication should be used cautiously in patients with liver disease. Patients who have experienced jaundice with a phenothiazine should not, if possible, be reexposed to chlorpromazine or other phenothiazines.
If fever with grippe-like symptoms occurs, appropriate liver studies should be conducted. If tests indicate an abnormality, stop treatment.
Liver function tests in jaundice induced by the drug may mimic extrahepatic obstruction; withhold exploratory laparotomy until extrahepatic obstruction is confirmed.
Most cases have occurred between the fourth and tenth weeks of therapy; patients should be watched closely during that period.
Moderate suppression of white blood cells is not an indication for stopping treatment unless accompanied by the symptoms described above.
To control hypotension, place patient in head-low position with legs raised. If a vasoconstrictor is required, norepinephrine and phenylephrine are the most suitable. Other pressor agents, including epinephrine, should not be used as they may cause a paradoxical further lowering of blood pressure.
If these symptoms become too troublesome, they can usually be controlled by a reduction of dosage or change of drug. Treatment with anti-parkinsonian agents, benzodiazepines or propranolol may be helpful.
There is no known effective treatment for tardive dyskinesia; anti-parkinsonism agents do not alleviate the symptoms of this syndrome. If clinically feasible, it is suggested that all antipsychotic agents be discontinued if these symptoms appear. Should it be necessary to reinstitute treatment, or increase the dosage of the agent, or switch to a different antipsychotic agent, the syndrome may be masked.
It has been reported that fine vermicular movements of the tongue may be an early sign of the syndrome and, if the medication is stopped at that time, the syndrome may not develop.
Cerebral edema has been reported.
Convulsive seizures (petit mal and grand mal) have been reported, particularly in patients with EEG abnormalities or history of such disorders.
Abnormality of the cerebrospinal fluid proteins has also been reported.
Contact dermatitis has been reported in nursing personnel; accordingly, the use of rubber gloves when administering chlorpromazine liquid or injectable is recommended.
In addition, asthma, laryngeal edema, angioneurotic edema and anaphylactoid reactions have been reported.
The pigmentary changes, restricted to exposed areas of the body, range from an almost imperceptible darkening of the skin to a slate gray color, sometimes with a violet hue. Histological examination reveals a pigment, chiefly in the dermis, which is probably a melanin-like complex. This pigmentation may fade following discontinuance of the drug.
Since the occurrence of eye changes seems to be related to dosage levels and/or duration of therapy, it is suggested that long-term patients on moderate to high dosage levels have periodic ocular examinations.
Chlorpromazine hydrochloride, USP, a dimethylamine derivative of phenothiazine, has a chemical formula of 2-chloro-10-[3-(dimethylamino) propyl] phenothiazine monohydrochloride. It is available in tablets for oral administration. It has the following structural formula:
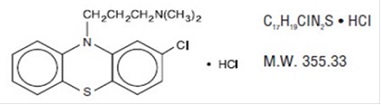
Chlorpromazine hydrochloride, USP occurs as white to creamy white, crystalline powder.
Each tablet for oral administration contains 10 mg, 25 mg, 50 mg, 100 mg, or 200 mg of chlorpromazine hydrochloride, USP.
Inactive ingredients: lactose monohydrate, crospovidone, sodium starch glycolate (10 mg and 25 mg), microcrystalline cellulose (50 mg, 100 mg and 200 mg) and sodium lauryl sulfate (50 mg, 100 mg and 200 mg) povidone K-30, magnesium stearate, hypromellose, titanium dioxide, talc, polyethylene glycol 6000 (10 mg and 25 mg) and polyethylene glycol 3350 (50 mg, 100 mg and 200 mg).
Additionally, 50 mg, 100 mg and 200 mg
The tablets are imprinted with edible black ink. The edible black ink contains shellac, black iron oxide and propylene glycol.
FDA approved dissolution test specifications differ from USP.