Chlorthalidone
Chlorthalidone Prescribing Information
Diuretics such as chlorthalidone are indicated in the management of hypertension either as the sole therapeutic agent or to enhance the effect of other antihypertensive drugs in the more severe forms of hypertension.
Chlorthalidone is indicated as adjunctive therapy in edema associated with congestive heart failure, hepatic cirrhosis, and corticosteroid and estrogen therapy.
Chlorthalidone has also been found useful in edema due to various forms of renal dysfunction, such as nephrotic syndrome, acute glomerulonephritis, and chronic renal failure.
Edema during pregnancy may arise from pathological causes or from the physiologic and mechanical consequences of pregnancy. Chlorthalidone is indicated in pregnancy when edema is due to pathologic causes, just as it is in the absence of pregnancy (however, see
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Digitalis therapy may exaggerate metabolic effects of hypokalemia especially with reference to myocardial activity.
Any chloride deficit is generally mild and usually does not require specific treatment except under extraordinary circumstances (as in liver disease or renal disease).
Dilutional hyponatremia may occur in edematous patients in hot weather, appropriate therapy is water restriction, rather than administration of salt except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving chlorthalidone. Thiazide-like diuretics have been shown to increase the urinary excretion of magnesium; this may result in hypomagnesemia.
The antihypertensive effects of the drug may be enhanced in the post-sympathectomy patient.
If progressive renal impairment becomes evident, as indicated by a rising nonprotein nitrogen or blood urea nitrogen, a careful reappraisal of therapy is necessary with consideration given to withholding or discontinuing diuretic therapy.
Calcium excretion is decreased by thiazide-like drugs. Pathological changes in the parathyroid gland with hypercalcemia and hypophosphatemia have been observed in few patients on thiazide therapy. The common complications of hyperparathyroidism such as renal lithiasis, bone resorption and peptic ulceration have not been seen.
Patients should be cautioned to contact their physician if they experience any of the following symptoms of potassium loss: excess thirst, tiredness, drowsiness, restlessness, muscle pains or cramps, nausea, vomiting, or increased heart rate or pulse.
Patients should also be cautioned that taking alcohol can increase the chance of dizziness occurring.
All patients receiving chlorthalidone should be observed for clinical signs of fluid or electrolyte imbalance: namely, hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids.
Medication such as digitalis may also influence serum electrolytes. Warning signs, irrespective of cause, are: Dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Insulin requirements in diabetic patients may be increased, decreased, or unchanged. Higher dosage of oral hypoglycemic agents may be required. Latent diabetes mellitus may become manifest during chlorthalidone administration.
Chlorthalidone and related drugs may increase the responsiveness to tubocurarine.
Chlorthalidone and related drugs may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
Therapy should be initiated with the lowest possible dose. This dose should be titrated according to individual patient response to gain maximal therapeutic benefit while maintaining lowest dosage possible. A single dose given in the morning with food is recommended; divided daily doses are unnecessary.
Anuria.
Known hypersensitivity to chlorthalidone or other sulfonamide-derived drugs.
The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency.
anorexia,
gastric irritation
nausea,
vomiting
cramping
diarrhea
constipation
jaundice (intrahepatic cholestatic jaundice)
pancreatitis
dizziness
vertigo
paresthesias
headache
xanthopsia
leukopenia
agranulocytosis
thrombocytopenia
aplastic anemia
purpura
photosensitivity
rash
urticaria
necrotizing angiitis
(vasculitis)
(cutaneous vasculitis)
Lyell's syndrome (toxic epidermal necrolysis)
hyperglycemia
glycosuria
hyperuricemia
muscle spasm
weakness
restlessness
impotence
Whenever adverse reactions are moderate or severe, chlorthalidone dosage should be reduced or therapy withdrawn.
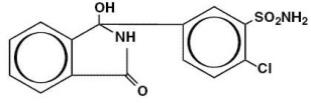
Chlorthalidone USP is an oral antihypertensive/diuretic. It is a monosulfamyl diuretic that differs chemically from thiazide diuretics in that a double-ring system is incorporated in its structure. It is 2-chloro-5(1-hydroxy-3-oxo-1-isoindolinyl) benzenesulfonamide, with the following structural formula:
Chlorthalidone is an oral diuretic with prolonged action (48–72 hours) and low toxicity. The major portion of the drug is excreted unchanged by the kidneys. The diuretic effect of the drug occurs in approximately 2.6 hours and continues for up to 72 hours. The mean half-life following a 50 to 200 mg dose is 40 hours. In the first order of absorption, the elimination half-life is 53 hours following a 50 mg dose, and 60 hours following a 100 mg dose. Approximately 75 percent of the drug is bound to plasma proteins, 58 percent of the drug being bound to albumin. This is caused by an increased affinity of the drug to erythrocyte carbonic anhydrase. Nonrenal routes of elimination have yet to be clarified. Data are not available regarding percentage of dose as unchanged drug and metabolites, concentration of the drug in body fluids, degree of uptake by a particular organ or in the fetus, or passage across the blood-brain barrier.
The drug produces copious diuresis with greatly increased excretion of sodium and chloride. At maximal therapeutic dosage, chlorthalidone is approximately equal in its diuretic effect to comparable maximal therapeutic doses of benzothiadiazine diuretics. The site of action appears to be the cortical diluting segment of the ascending limb of Henle's loop of the nephron.