Cholestyramine For Oral Suspension
Cholestyramine For Oral Suspension Prescribing Information
1) Cholestyramine is indicated as adjunctive therapy to diet for the reduction of elevated serum cholesterol in patients with primary hypercholesterolemia (elevated low density lipoprotein [LDL] cholesterol) who do not respond adequately to diet. Cholestyramine may be useful to lower LDL cholesterol in patients who also have hypertriglyceridemia, but it is not indicated where hypertriglyceridemia is the abnormality of most concern.
Therapy with lipid-altering agents should be a component of multiple risk factor intervention in those individuals at significantly increased risk for atherosclerotic vascular disease due to hypercholesterolemia. Treatment should begin and continue with dietary therapy specific for the type of hyperlipoproteinemia determined prior to initiation of drug therapy. Excess body weight may be an important factor and caloric restriction for weight normalization should be addressed prior to drug therapy in the overweight.
Prior to initiating therapy with cholestyramine resin, secondary causes of hypercholesterolemia (e.g., poorly controlled diabetes mellitus, hypothyroidism, nephrotic syndrome, dysproteinemias, obstructive liver disease, other drug therapy, alcoholism), should be excluded and a lipid profile performed to assess Total cholesterol, HDL-C and triglycerides (TG). For individuals with TG less than 400 mg/dL (<4.5 mmol/L), LDL-C can be estimated using the following equation:
LDL-C = Total cholesterol - [(TG/5) + HDL-C]
For TG levels > 400 mg/dL, this equation is less accurate and LDL-C concentrations should be determined by ultracentrifugation. In hypertriglyceridemic patients, LDL-C may be low or normal despite elevated Total-C. In such cases cholestyramine resin may not be indicated.
Serum cholesterol and triglyceride levels should be determined periodically based on NCEP guidelines to confirm initial and adequate long-term response. A favorable trend in cholesterol reduction should occur during the first month of cholestyramine resin therapy. The therapy should be continued to sustain cholesterol reduction. If adequate cholesterol reduction is not attained, increasing the dosage of cholestyramine resin or adding other lipid-lowering agents in combination with cholestyramine resin should be considered.
Since the goal of treatment is to lower LDL-C, the NCEP4 recommends that LDL-C levels be used to initiate and assess treatment response. If LDL-C levels are not available then Total-C alone may be used to monitor long-term therapy. A lipoprotein analysis (including LDL-C determination) should be carried out once a year. The NCEP treatment guidelines are summarized below.
Definite Atherosclerotic Disease * | Two or More Other Risk Factors † | LDL-Cholesterol mg/dL (mmol/L) | |
Initiation Level | Goal | ||
No | No | ≥ 190 (4.9) | ≥ 60 (4.1) |
No | Yes | ≥ 160 (4.1) | ≥ 130 (3.4) |
Yes | Yes or No | ≥ 130 (3.4) | ≥ 100 (‑2.6) |
*Coronary heart disease or peripheral vascular disease (including symptomatic carotid artery disease).
†Other risk factors for coronary heart disease (CHD) include: age (males 45 years; females: 55 years or premature menopause without estrogen replacement therapy); family history of premature CHD; current cigarette smoking; hypertension; confirmed HDL-C <35 mg/dL (<0.91 mmol/L); and diabetes mellitus. Subtract one risk factor if HDL-C is 60 mg/dL (1.6 mmol/L).
Cholestyramine resin monotherapy has been demonstrated to retard the rate of progression
2,3 and increase the rate of regression
3 of coronary atherosclerosis.
2) Cholestyramine is indicated for the relief of pruritus associated with partial biliary obstruction. Cholestyramine resin has been shown to have a variable effect on serum cholesterol in these patients. Patients with primary biliary cirrhosis may exhibit an elevated cholesterol as part of their disease.
The color of cholestyramine for oral suspension products powder may vary somewhat from batch to batch but this variation does not affect the performance of the product. Place the contents of one level scoopful of cholestyramine for oral suspension products powder in a glass or cup. Add at least 2 to 3 ounces of water or the beverage of your choice. Stir to a uniform consistency.
Cholestyramine for oral suspension products powder may also be mixed with highly fluid soups or pulpy fruits with a high moisture content such as applesauce or crushed pineapple.
Cholestyramine for oral suspension and cholestyramine for oral suspension light is contraindicated in patients with complete biliary obstruction where bile is not secreted into the intestine and in those individuals who have shown hypersensitivity to any of its components.
The most common adverse reaction is constipation. When used as a cholesterol-lowering agent predisposing factors for most complaints of constipation are high dose and increased age (more than 60 years old). Most instances of constipation are mild, transient and controlled with conventional therapy. Some patients require a temporary decrease in dosage or discontinuation of therapy.
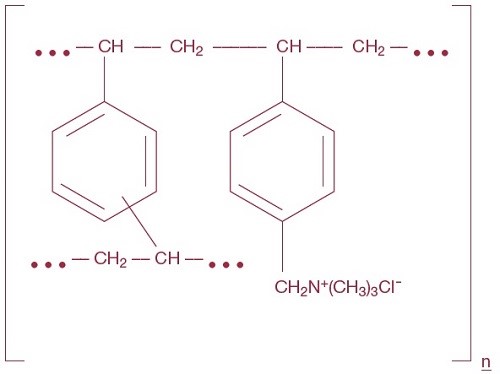
Cholestyramine, the chloride salt of a basic anion exchange resin, a cholesterol lowering agent, is intended for oral administration. Cholestyramine resin is quite hydrophilic, but insoluble in water. Cholestyramine resin is not absorbed from the digestive tract. Each 9 grams of Cholestyramine for Oral Suspension, USP powder contains 4 grams of cholestyramine resin. Each 5.7 grams of Cholestyramine for Oral Suspension, USP Light powder contains 4 grams of cholestyramine resin. It is represented by the following structural formula:
Cholesterol is probably the sole precursor of bile acids. During normal digestion, bile acids are secreted into the intestines. A major portion of the bile acids is absorbed from the intestinal tract and returned to the liver via the enterohepatic circulation. Only very small amounts of bile acids are found in normal serum.
Cholestyramine resin adsorbs and combines with the bile acids in the intestine to form an insoluble complex which is excreted in the feces. This results in a partial removal of bile acids from the enterohepatic circulation by preventing their absorption.
The increased fecal loss of bile acids due to cholestyramine resin administration leads to an increased oxidation of cholesterol to bile acids, a decrease in beta lipoprotein or low density lipoprotein plasma levels and a decrease in serum cholesterol levels. Although in man, cholestyramine resin produces an increase in hepatic synthesis of cholesterol, plasma cholesterol levels fall.
In patients with partial biliary obstruction, the reduction of serum bile acid levels by cholestyramine resin reduces excess bile acids deposited in the dermal tissue with resultant decrease in pruritus.