Choletec
(Mebrofenin)Choletec Prescribing Information
Technetium Tc 99m Mebrofenin is indicated as a hepatobiliary imaging agent.
The suggested intravenous dose range of Technetium Tc 99m Mebrofenin in the average patient (70 kg) is:
| Nonjaundiced patient: | 74-185 MBq (2-5 mCi) |
| Patient with serum bilirubin level greater than 1.5 mg/dL: | 111-370 MBq (3-10 mCi) |
The patient dose should be measured by a suitable radioactivity calibration system immediately prior to administration.
The patient should be in a fasting state, 4 hours is preferable. False positives (non-visualization) may result if the gallbladder has been emptied by ingestion of food.
An interval of at least 24 hours should be allowed before repeat examination.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Hypersensitivity to this compound.
Urticaria and rash have been rarely reported with the use of Technetium Tc 99m Mebrofenin since market introduction. Rare cases of chills and nausea have been reported with related compounds. Infrequently, death has been reported in association with the use of this class of agents.
Each reaction vial contains a nonradioactive, sterile, nonpyrogenic mixture of 45 mg mebrofenin, 0.54 mg (minimum) stannous fluoride dihydrate, SnF2•2H2O and 1.03 mg total tin, maximum (as stannous fluoride dihydrate, SnF2•2H2O), not more than 5.2 mg methylparaben, and 0.58 mg propylparaben. The pH is adjusted with sodium hydroxide or hydrochloric acid prior to lyophilization. The contents of the vial are lyophilized and sealed under nitrogen at the time of manufacture.
The pH of the reconstituted product is 4.2 to 5.7.
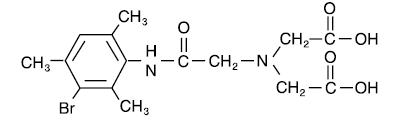
The structure of mebrofenin (2,2’-[[2-[(3-Bromo-2,4,6-trimethylphenyl)-amino]-2-oxoethyl]imino] bisacetic acid) is shown below:
When sterile, pyrogen-free sodium pertechnetate Tc 99m injection is added to the vial, the diagnostic agent Technetium Tc 99m Mebrofenin is formed for administration by intravenous injection.
Mebrofenin is an iminodiacetic acid (HIDA) derivative with no known pharmacologic action at the recommended doses.
Following intravenous administration in normal subjects, Technetium Tc 99m Mebrofenin was rapidly cleared from the circulation. The mean percent injected dose remaining in the blood at 10 minutes was 17%. The injected activity was cleared through the hepatobiliary system with visualization of the liver by 5 minutes and maximum liver uptake occurring at 11 minutes post-injection. Hepatic duct and gallbladder visualization occurred by 10 to 15 minutes and intestinal activity was visualized by 30 to 60 minutes in subjects with normal hepatobiliary function. The mean percent injected dose excreted in the urine during the first 3 hours was 1% (0.4 to 2.0%).
Elevated serum bilirubin levels increase renal excretion of Tc 99m HIDA agents. In two studies in which Tc 99m Mebrofenin was administered to patients having mean elevated serum bilirubin levels of 9.8 mg/dL (1.7 to 46.3 mg/dL), the mean percent injected dose excreted in the urine during the first 3 hours was 3% (0.2 to 11.5%). The mean percent injected dose excreted in the urine during 3-24 hours was 14.9% (0.4 to 34.8%).
In jaundiced patients, the percent injected dose remaining in the blood at 10 minutes may be twice as high or more than the level in normals. Hepatobiliary transit may be delayed and visualization times increased. As a consequence, the quality of the images obtained frequently diminishes.