Ciprofloxacin Hydrochloride
Ciprofloxacin Hydrochloride Prescribing Information
Ciprofloxacin ophthalmic Solution is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
*Efficacy for this organism was studied in fewer than 10 infections.
A history of hypersensitivity to ciprofloxacin or any other component of the medication is a contraindication to its use. A history of hypersensitivity to other quinolones may also contraindicate the use of ciprofloxacin.
The most frequently reported drug related adverse reaction was local burning or discomfort. In corneal ulcer studies with frequent administration of the drug, white crystalline precipitates were seen in approximately 17% of patients (SEE
Ciprofloxacin should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity reaction.
In clinical studies of patients with bacterial corneal ulcer, a white crystalline precipitate located in the superficial portion of the corneal defect was observed in 35 (16.6%) of 210 patients. The onset of the precipitate was within 24 hours to 7 days after starting therapy. In one patient, the precipitate was immediately irrigated out upon its appearance. In 17 patients, resolution of the precipitate was seen in 1 to8 days (seven within the first 24 to 72 hours), in five patients, resolution was noted in 10 to 13 days. In nine patients, exact resolution days were unavailable; however, at follow-up examinations, 18 to 44 days after onset of the event, complete resolution of the precipitate was noted. In three patients, outcome information was unavailable. The precipitate did not preclude continued use of ciprofloxacin, nor did it adversely affect the clinical course of the ulcer or visual outcome. (SEE
Mouse Lymphoma Cell Forward Mutation Assay (Positive)
Chinese Hamster V79 Cell HGPRT Test (Negative)
Syrian Hamster Embryo Cell Transformation Assay (Negative)
Rat Hepatocyte DNA Repair Assay (Positive)
Thus, two of the eight tests were positive, but the results of the following three
Rat Hepatocyte DNA Repair Assay
Micronucleus Test (Mice)
Dominant Lethal Test (Mice)
Long term carcinogenicity studies in mice and rats have been completed. After daily oral dosing for up to two years, there is no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species.
Ciprofloxacin Ophthalmic Solution should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
The safety and effectiveness of ciprofloxacin ophthalmic solution 0.3% have been established in all ages. Use of ciprofloxacin is supported by evidence from adequate and well controlled studies of ciprofloxacin in adults, children and neonates
Ciprofloxacin ophthalmic solution is a synthetic, sterile, multiple dose, antimicrobial for topical ophthalmic use. Ciprofloxacin is a fluoroquinolone antibacterial active against a broad spectrum of gram-positive and gram-negative ocular pathogens. It is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-quinoline-carboxylic acid. It is a faint to light yellow crystalline powder with a molecular weight of 385.8. Its empirical formula is C
17H
18FN
3O
3•HCL•H
2O and its chemical structure is as follows:
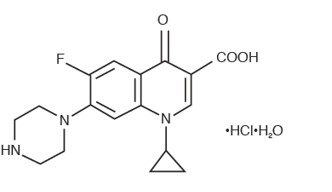
Ciprofloxacin differs from other quinolones in that it has a fluorine atom at the 6-position, a piperazine moiety at the 7-position, and a cyclopropyl ring at the 1-position.
Each mL of ciprofloxacin ophthalmic solution contains:
Ciprofloxacin has been shown to be active against most strains of the following organisms both
Ciprofloxacin ophthalmic Solution is indicated for the treatment of infections caused by susceptible strains of the designated microorganisms in the conditions listed below:
*Efficacy for this organism was studied in fewer than 10 infections.
Ciprofloxacin has been shown to be active
Most strains of
Ciprofloxacin does not cross-react with other antimicrobial agents such as beta-lactams or aminoglycosides; therefore, organisms resistant to these drugs may be susceptible to ciprofloxacin.