Clarithromycin - Clarithromycin tablet, Film Coated, Extended Release prescribing information
Clarithromycin is a macrolide antimicrobial indicated for mild to moderate infections caused by designated, susceptible bacteria in the following:
- Acute Bacterial Exacerbation of Chronic Bronchitis in Adults ()
1.1 Acute Bacterial Exacerbation
of Chronic BronchitisClarithromycin extended-release tablets are indicated in adults for the treatment of mild to moderate infections caused by susceptible isolates due to
Haemophilus influenzae, Haemophilus parainfluenzae, Moraxella catarrhalis,orStreptococcus pneumoniae [see Indications and Usage ]. - Acute Maxillary Sinusitis ()
1.2 Acute Maxillary SinusitisClarithromycin extended-release tablets (in adults) are indicated for the treatment of mild to moderate infections caused by susceptible isolates due to
Haemophilus influenzae, Moraxella catarrhalis,orStreptococcus pneumoniae [see Indications and Usage ]. - Community-Acquired Pneumonia ()
1.3 Community-Acquired
PneumoniaClarithromycin extended-release tablets are indicated
[see Indications and Usage ]for the treatment of mild to moderate infections caused by susceptible isolates due to:- Haemophilus influenzae(in adults)
- Haemophilus parainfluenzae(in adults)
- Moraxella catarrhalis(in adults)
- Mycoplasma pneumoniae, Streptococcus pneumoniae,Chlamydophilapneumoniae(in adults)
Clarithromycin extended-release tablets are indicated only for acute bacterial exacerbation of chronic bronchitis, acute maxillary sinusitis, and community-acquired pneumonia in adults. (
Clarithromycin extended-release tablets are indicated only for acute maxillary sinusitis, acute bacterial exacerbation of chronic bronchitis, and community-acquired pneumonia in adults. The efficacy and safety of clarithromycin extended-release tablets in treating other infections for which clarithromycin immediate-release tablets and clarithromycin granules are approved have not been established.
There is resistance to macrolides in certain bacterial infections caused by
To reduce the development of drug-resistant bacteria and maintain the effectiveness of clarithromycin and other antibacterial drugs, clarithromycin should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (
Clarithromycin extended-release tablets are indicated only for acute maxillary sinusitis, acute bacterial exacerbation of chronic bronchitis, and community-acquired pneumonia in adults. The efficacy and safety of clarithromycin extended-release tablets in treating other infections for which clarithromycin immediate-release tablets and clarithromycin granules are approved have not been established.
There is resistance to macrolides in certain bacterial infections caused by
- Adults: Clarithromycin extended-release tablets 1 gram every 24 hours for 7 to 14 days ()
2.2 Adult DosageThe recommended dosages of clarithromycin extended-release tablets for the treatment of mild to moderate infections in adults are listed in Table 1.
Table 1. Adult Dosage Guidelines Clarithromycin Extended-Release TabletsInfectionDosage(every 24 hours)Duration(days)Acute bacterial exacerbation of chronic bronchitis
1 gram
7
Acute maxillary sinusitis
1 gram
14
Community-acquired pneumonia
1 gram
7
- Reduce dose in moderate renal impairment with concomitant atazanavir or ritonavir-containing regimens and in severe renal impairment ()
2.6 Dosage Adjustment
in Patients with Renal ImpairmentSee Table 2 for dosage adjustment in patients with moderate or severe renal impairment with or without concomitant atazanavir or ritonavir-containing regimens
[see Drug Interactions ].Table 2. Clarithromycin Dosage Adjustments in Patients with Renal Impairment RecommendedClarithromycinDosage ReductionPatients with severe renal impairment (CLcr of <30 mL/min)
Reduce the dosage of
Clarithromycin by 50%
Patients with moderate renal impairment (CLcr of 30 to 60 mL/min) taking concomitant atazanavir or ritonavir-containing regimens
Reduce the dosage of
Clarithromycin by 50%
Patients with severe renal impairment (CLcr of <30 mL/min) taking concomitant atazanavir or ritonavir-containing regimens
Reduce the dosage of
Clarithromycin by 75%
Clarithromycin Extended-release Tablets USP (yellow, film coated, oval shaped, unscored tablets) are available as:
• 500 mg: debossed with 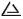 and “777” on one side.
and “777” on one side.
Geriatric: Increased risk of
In a steady-state study in which healthy elderly subjects (65 years to 81 years of age) were given 500 mg of clarithromycin every 12 hours, the maximum serum concentrations and area under the curves of clarithromycin and 14-OH clarithromycin were increased compared to those achieved in healthy young adults. These changes in pharmacokinetics parallel known age-related decreases in renal function. In clinical trials, elderly patients did not have an increased incidence of adverse reactions when compared to younger patients. Consider dosage adjustment in elderly patients with severe renal impairment. Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients
Most reports of acute kidney injury with calcium channel blockers metabolized by CYP3A4 (e.g., verapamil, amlodipine, diltiazem, nifedipine) involved elderly patients 65 years of age or older
Especially in elderly patients, there have been reports of colchicine toxicity with concomitant use of clarithromycin and colchicine, some of which occurred in patients with renal insufficiency. Deaths have been reported in some patients
The following serious adverse reactions are described below and elsewhere in the labeling:
- Acute Hypersensitivity Reactions [see Warnings and Precautions ]
- QT Prolongation [see Warnings and Precautions ]
- Hepatotoxicity [see Warnings and Precautions ]
- Serious Adverse Reactions Due to Concomitant Use with Other Drugs [see Warnings and Precautions ]
- Clostridium difficileAssociated Diarrhea[see Warnings and Precautions ]
- Exacerbation of Myasthenia Gravis [see Warnings and Precautions ]
Co-administration of clarithromycin is known to inhibit CYP3A, and a drug primarily metabolized by CYP3A may be associated with elevations in drug concentrations that could increase or prolong both therapeutic and adverse effects of the concomitant drug.
Clarithromycin should be used with caution in patients receiving treatment with other drugs known to be CYP3A enzyme substrates, especially if the CYP3A substrate has a narrow safety margin (e.g., carbamazepine) and/or the substrate is extensively metabolized by this enzyme. Adjust dosage when appropriate and monitor serum concentrations of drugs primarily metabolized by CYP3A closely in patients concurrently receiving clarithromycin.
Drugs That Are Affected By Clarithromycin | ||
Drug(s) with P h armacokinetics Affected by Clarithromycin | Recommendation | Comments |
Antiarrhythmics: Disopyramide | Not | Disopyramide, Quinidine: There have been postmarketing reports of torsades de pointes occurring with concurrent use of clarithromycin and quinidine or disopyramide. Electrocardiograms should be monitored for QTc prolongation during co-administration of clarithromycin with these drugs [see Warnings and Precautions ] .Serum concentrations of these medications should also be monitored. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with disopyramide and quinidine. There have been postmarketing reports of hypoglycemia with the concomitant administration of clarithromycin and disopyramide. Therefore, blood glucose levels should be monitored during concomitant administration of clarithromycin and disopyramide. |
Digoxin | Use With Caution | Digoxin: Digoxin is a substrate for P-glycoprotein (Pgp) and clarithromycin is known to inhibit Pgp. When clarithromycin and digoxin are co-administered, inhibition of Pgp by clarithromycin may lead to increased exposure of digoxin. Elevated digoxin serum concentrations in patients receiving clarithromycin and digoxin concomitantly have been reported in postmarketing surveillance. Some patients have shown clinical signs consistent with digoxin toxicity, including potentially fatal arrhythmias. Monitoring of serum digoxin concentrations should be considered, especially for patients with digoxin concentrations in the upper therapeutic range. |
Oral Anticoagulants: Warfarin | Use With Caution | Oral anticoagulants: Spontaneous reports in the postmarketing period suggest that concomitant administration of clarithromycin and oral anticoagulants may potentiate the effects of the oral anticoagulants. Prothrombin times should be carefully monitored while patients are receiving clarithromycin and oral anticoagulants simultaneously [see Warnings and Precautions ] . |
Antiepileptics: Carbamazepine | Use With Caution | C ar bamazepine: Concomitant administration of single doses of clarithromycin and carbamazepine has been shown to result in increased plasma concentrations of carbamazepine. Blood level monitoring of carbamazepine may be considered. Increased serum concentrations of carbamazepine were observed in clinical trials with clarithromycin. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with carbamazepine. |
Antifungals: Itraconazole | Use With Caution | I traconazole: Both clarithromycin and itraconazole are substrates and inhibitors of CYP3A, potentially leading to a bi-directional drug interaction when administered concomitantly (see also Itraconazole under “Drugs That Affect Clarithromycin” in the table below). Clarithromycin may increase the plasma concentrations of itraconazole. Patients taking itraconazole and clarithromycin concomitantly should be monitored closely for signs or symptoms of increased or prolonged adverse reactions. |
Fluconazole | No Dose Adjustment | F luconazole: [see Pharmacokinetics ] |
Anti-Gout Agents: Colchicine (in patients with renal or hepatic impairment) Colchicine (in patients with normal renal and hepatic function) | Contraindicated Use With Caution | C olchicine: Colchicine is a substrate for both CYP3A and the efflux transporter, P-glycoprotein (Pgp). Clarithromycin and other macrolides are known to inhibit CYP3A and Pgp. The dose of colchicine should be reduced when co-administered with clarithromycin in patients with normal renal and hepatic function [see Contraindications and Warnings and Precautions ] . |
Antipsychotics: Pimozide Quetiapine Lurasidone | Contraindicated | P imozide: [See Contraindications ] Quetiapine: Quetiapine is a substrate for CYP3A4, which is inhibited by clarithromycin. Co-administration with clarithromycin could result in increased quetiapine exposure and possible quetiapine related toxicities. There have been postmarketing reports of somnolence, orthostatic hypotension, altered state of consciousness, neuroleptic malignant syndrome, and QT prolongation during concomitant administration. Refer to quetiapine prescribing information for recommendations on dose reduction if co-administered with CYP3A4 inhibitors such as clarithromycin.Lurasidone : [See Contraindications ( Concomitant administration of clarithromycin and lurasidone is contraindicated since it may result in an increase in lurasidone exposure and the potential for serious adverse reactions [see Drug Interactions ] . |
Antispasmodics: Tolterodine (patients deficient in CYP2D6 activity) | Use With Caution | Tolterodine: The primary route of metabolism for tolterodine is via CYP2D6. However, in a subset of the population devoid of CYP2D6, the identified pathway of metabolism is via CYP3A. In this population subset, inhibition of CYP3A results in significantly higher serum concentrations of tolterodine. Tolterodine 1 mg twice daily is recommended in patients deficient in CYP2D6 activity (poor metabolizers) when co-administered with clarithromycin. |
Antivirals: Atazanavir | Use With Caution | Atazanavir: Both clarithromycin and atazanavir are substrates and inhibitors of CYP3A, and there is evidence of a bi-directional drug interaction (see Atazanavir under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics ] . |
Saquinavir (in patients with decreased renal function) | S a quinavir: Both clarithromycin and saquinavir are substrates and inhibitors of CYP3A and there is evidence of a bi-directional drug interaction (see Saquinavir under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics ] . | |
Ritonavir Etravirine | R itonavir, Etravirine: (see Ritonavir and Etravirine under “Drugs That Affect Clarithromycin” in the table below) [see Pharmacokinetics ] . | |
Maraviroc | Maraviroc: Clarithromycin may result in increases in maraviroc exposures by inhibition of CYP3A metabolism. See Selzentry® prescribing information for dose recommendation when given with strong CYP3A inhibitors such as clarithromycin. | |
Boceprevir (in patients with normal renal function) Didanosine | No Dose Adjustment | B oceprevir: Both clarithromycin and boceprevir are substrates and inhibitors of CYP3A, potentially leading to a bi-directional drug interaction when co-administered. No dose adjustments are necessary for patients with normal renal function (see Victrelis® prescribing information). |
Zidovudine | Z idovudine: Simultaneous oral administration of clarithromycin immediate-release tablets and zidovudine to HIV-infected adult patients may result in decreased steady-state zidovudine concentrations. Administration of clarithromycin and zidovudine should be separated by at least two hours [see Pharmacokinetics ] .The impact of co-administration of clarithromycin extended-release tablets or granules and zidovudine has not been evaluated. | |
Calcium Channel Verapamil Amlodipine Nifedipine | Use With Caution | Verapamil: Hypotension, bradyarrhythmias, and lactic acidosis have been observed in patients receiving concurrent verapamil, [see Warnings and Precautions ] .Amlodipine, Diltiazem: [See Warnings and Precautions ] Nifedipine: Nifedipine is a substrate for CYP3A. Clarithromycin and other macrolides are known to inhibit CYP3A. There is potential of CYP3A-mediated interaction between nifedipine and clarithromycin. Hypotension and peripheral edema were observed when clarithromycin was taken concomitantly with nifedipine [see Warnings and Precautions ] . |
Ergot Alkaloids: Ergotamine | Contraindicated | Ergotamine, Dihydroergotamine: Postmarketing reports indicate that co-administration of clarithromycin with ergotamine or dihydroergotamine has been associated with acute ergot toxicity characterized by vasospasm and ischemia of the extremities and other tissues including the central nervous system [see Contraindications ] . |
Gastroprokinetic Cisapride | Contraindicated | C isapride: [See Contraindications ] |
Lipid-lowering agents: Lomitapide Atorvastatin | Contraindicated Use With Caution No Dose | Lomitapide, L ovastatin, Simvastatin: Clarithromycin may increase the exposure of these drugs by inhibition of CYP3A metabolism, thereby increasing the risk of toxicities from these drugs [see Contraindications and Warnings and Precautions ] Atorvastatin, Pravastatin, Fluvastatin: [See Warnings and Precautions ] |
Hypoglycemic Agents: Nateglinide Pioglitazone Repaglinide Rosiglitazone Insulin | Use With Caution | Nateglinide, Pioglitazone, Repaglinide, Rosiglitazone: [See Warnings and Precautions and Adverse Reactions ] I nsulin: [See Warnings and Precautions and Adverse Reactions ] |
Immunosuppressants: Cyclosporine Tacrolimus | Use With Caution | C y c losporine: There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with cyclosporine.Tacrolimus: There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with tacrolimus. |
Phosphodiesterase inhibitors: Sildenafil | Use With Caution | S ildenafil, Tadalafil, Vardenafil: Each of these phosphodiesterase inhibitors is primarily metabolized by CYP3A, and CYP3A will be inhibited by concomitant administration of clarithromycin. Co-administration of clarithromycin with sildenafil, tadalafil, or vardenafil will result in increased exposure of these phosphodiesterase inhibitors. Co-administration of these phosphodiesterase inhibitors with clarithromycin is not recommended. Increased systemic exposure of these drugs may occur with clarithromycin; reduction of dosage for phosphodiesterase inhibitors should be considered (see their respective prescribing information). |
Proton Pump Inhibitors: Omeprazole | No Dose | Omeprazole: The mean 24-hour gastric pH value was 5.2 when omeprazole was administered alone and 5.7 when co-administered with clarithromycin as a result of increased omeprazole exposures [see Pharmacokinetics ] (see also Omeprazole under “Drugs That Affect Clarithromycin” in the table below). |
Xanthine Derivatives: Theophylline | Use With Caution | Theophylline: Clarithromycin use in patients who are receiving theophylline may be associated with an increase of serum theophylline concentrations [see Pharmacokinetics ] . Monitoring of serum theophylline concentrations should be considered for patients receiving high doses of theophylline or with baseline concentrations in the upper therapeutic range. |
Triazolobenzodiazepines and Other Related Benzodiazepines: Midazolam Alprazolam Temazepam | Use With Caution No Dose | Midazolam: When oral midazolam is co-administered with clarithromycin, dose adjustments may be necessary and possible prolongation and intensity of effect should be anticipated [see Warnings and Precautions and Pharmacokinetics ] .Triazolam, Alprazolam: Caution and appropriate dose adjustments should be considered when triazolam or alprazolam is co-administered with clarithromycin. There have been postmarketing reports of drug interactions and central nervous system (CNS) effects (e.g., somnolence and confusion) with the concomitant use of clarithromycin and triazolam. Monitoring the patient for increased CNS pharmacological effects is suggested.In postmarketing experience, erythromycin has been reported to decrease the clearance of triazolam and midazolam, and thus, may increase the pharmacologic effect of these benzodiazepines. Temazepam, Nitrazepam, Lorazepam: For benzodiazepines which are not metabolized by CYP3A (e.g., temazepam, nitrazepam, lorazepam), a clinically important interaction with clarithromycin is unlikely. |
Cytochrome P450 Rifabutin | Use With Caution | R ifabutin: Concomitant administration of rifabutin and clarithromycin resulted in an increase in rifabutin, and decrease in clarithromycin serum levels together with an increased risk of uveitis (see Rifabutin under “Drugs That Affect Clarithromycin” in the table below). |
Other Drugs Alfentanil | Use With Caution | There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with alfentanil, methylprednisolone, cilostazol, bromocriptine, vinblastine, phenobarbital, and St. John’s Wort. |
Other Drugs Metabolized by CYP450 Isoforms Other than CYP3A: Hexobarbital | Use With Caution | There have been postmarketing reports of interactions of clarithromycin with drugs not thought to be metabolized by CYP3A, including hexobarbital, phenytoin, and valproate. |
Drugs that Affect C larithromycin | ||
Drug(s) that Affect the Pharmacokinetics of C larithromycin | Recommendation | Comments |
Antifungals: Itraconazole | Use With Caution | I traconazole: Itraconazole may increase the plasma concentrations of clarithromycin. Patients taking itraconazole and clarithromycin concomitantly should be monitored closely for signs or symptoms of increased or prolonged adverse reactions (see also Itraconazole under “Drugs That Are Affected By Clarithromycin” in the table above). |
Antivirals: Atazanavir Ritonavir (in patients with decreased renal function) Saquinavir (in patients with decreased renal function) Etravirine Saquinavir (in patients with normal renal function) Ritonavir (in patients with normal renal function) | Use With Caution No Dose | Atazanavir: When clarithromycin is co-administered with atazanavir, the dose of clarithromycin should be decreased by 50% [see Clinical Pharmacology ( 12.3)] .Since concentrations of 14-OH clarithromycin are significantly reduced when clarithromycin is co-administered with atazanavir, alternative antibacterial therapy should be considered for indications other than infections due to Mycobacterium avium complex. Doses of clarithromycin greater than 1000 mg per day should not be co-administered with protease inhibitors.R itonavir: Since concentrations of 14-OH clarithromycin are significantly reduced when clarithromycin is co-administered with ritonavir, alternative antibacterial therapy should be considered for indications other than infections due to Mycobacterium avium [see Pharmacokinetics ] .Doses of clarithromycin greater than 1000 mg per day should not be co-administered with protease inhibitors. S a quinavir: When saquinavir is co-administered with ritonavir, consideration should be given to the potential effects of ritonavir on clarithromycin (refer to ritonavir above) [see Pharmacokinetics ] .Etravirine: Clarithromycin exposure was decreased by etravirine; however, concentrations of the active metabolite, 14-OH-clarithromycin, were increased. Because 14-OH-clarithromycin has reduced activity against Mycobacterium avium complex (MAC), overall activity against this pathogen may be altered; therefore alternatives to clarithromycin should be considered for the treatment of MAC. |
Proton Pump Inhibitors: Omeprazole | Use With Caution | Omeprazole: Clarithromycin concentrations in the gastric tissue and mucus were also increased by concomitant administration of omeprazole [see Pharmacokinetics ] . |
Miscellaneous Efavirenz | Use With Caution | Inducers of CYP3A enzymes, such as efavirenz, nevirapine, rifampicin, rifabutin, and rifapentine will increase the metabolism of clarithromycin, thus decreasing plasma concentrations of clarithromycin, while increasing those of 14-OH-clarithromycin. Since the microbiological activities of clarithromycin and 14-OH-clarithromycin are different for different bacteria, the intended therapeutic effect could be impaired during concomitant administration of clarithromycin and enzyme inducers. Alternative antibacterial treatment should be considered when treating patients receiving inducers of CYP3A. There have been spontaneous or published reports of CYP3A based interactions of clarithromycin with rifabutin (see Rifabutin under “Drugs That Are Affected By Clarithromycin” in the table above). |