Clemastine Fumarate
Clemastine Fumarate Prescribing Information
Clemastine Fumarate Tablets USP, 2.68 mg are indicated for the relief of symptoms associated with allergic rhinitis such as sneezing, rhinorrhea, pruritus, and lacrimation. Clemastine Fumarate Tablets USP, 2.68 mg are also indicated for the relief of mild, uncomplicated allergic skin manifestations of urticaria and angioedema. It should be noted that clemastine fumarate is indicated for the dermatologic indications at the 2.68 mg dosage level only.
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND RESPONSE OF THE PATIENT.
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Transient drowsiness, the most common adverse reaction associated with clemastine fumarate, occurs relatively frequently and may require discontinuation of therapy in some instances.
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Clemastine belongs to the benzhydryl ether group of antihistaminic compounds. The chemical name is (+)-(2
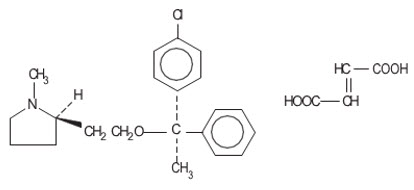 | |
| C21H26C1NO∙C4H4O4 | M.W. 459.97 |
Each tablet for oral administration contains 2.68 mg of clemastine fumarate, USP.
Inactive ingredients: colloidal silicon dioxide, corn starch, lactose monohydrate, povidone, pregelatinized starch and stearic acid.