Clobazam Prescribing Information
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death. Reserve concomitant prescribing of these drugs for patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation[see Warnings and Precautions (5.1), Drug Interactions (7.1)].
- The use of benzodiazepines, includingclobazam oral suspension, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing clobazam oral suspension and throughout treatment, assess each patient’s risk for abuse, misuse, and addiction[see Warnings and Precautions (5.2)].
- The continued use of benzodiazepines, including clobazam oral suspension, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of clobazam oral suspension after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue clobazam oral suspension or reduce the dosage[see Dosage and Administration (2.2)andWarnings and Precautions (5.3)].
Clobazam oral suspension is indicated for the adjunctive treatment of seizures associated with Lennox-Gastaut syndrome (LGS) in patients 2 years of age or older.
Oral Suspension: 2.5 mg/mL for oral administration. Each bottle contains 120 mL of an off-white suspension.
Clobazam oral suspension is contraindicated in patients with a history of hypersensitivity to the drug or its ingredients. Hypersensitivity reactions have included serious dermatological reactions
Serious skin reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported with clobazam in both children and adults during the postmarketing period. Patients should be closely monitored for signs or symptoms of SJS/TEN, especially during the first 8 weeks of treatment initiation or when re-introducing therapy. Clobazam should be discontinued at the first sign of rash, unless the rash is clearly not drug-related. If signs or symptoms suggest SJS/TEN, use of this drug should not be resumed and alternative therapy should be considered
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including clobazam. These events can be fatal or life-threatening, particularly if diagnosis and treatment do not occur as early as possible. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. clobazam should be discontinued if an alternative etiology for the signs or symptoms cannot be established
Clinically significant adverse reactions that appear in other sections of the labeling include the following:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- Abuse, Misuse, and Addiction [see Warnings and Precautions (5.2)]
- Dependence and Withdrawal Reactions [see Warnings and Precautions (5.3)]
- Potentiation of Sedation from Concomitant Use with Central Nervous System Depressants [see Warnings and Precautions (5.4)]
- Somnolence or Sedation [see Warnings and Precautions (5.5)]
- Serious Dermatological Reactions [see Contraindications (4), Warnings and Precautions (5.6)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see]
5.7 Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan HypersensitivityDrug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as multiorgan hypersensitivity, has been reported in patients taking antiepileptic drugs, including clobazam. These events can be fatal or life-threatening, particularly if diagnosis and treatment do not occur as early as possible. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis, sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. clobazam should be discontinued if an alternative etiology for the signs or symptoms cannot be established
[see Contraindications (4)]. - Suicidal Behavior and Ideation [see]
5.8 Suicidal Behavior and IdeationAntiepileptic drugs (AEDs), including clobazam, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted relative risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed. Table 2 shows absolute and relative risk by indication for all evaluated AEDs.
Table 2: Risk by Indication for Antiepileptic Drugs in the Pooled AnalysisIndicationPlacebo Patients with Events per 1,000 PatientsDrug Patients with Events per 1,000 PatientsRelative Risk: Incidence of Drug Events in Drug Patients/Incidence in Placebo PatientsRisk Difference: Additional Drug Patients with Events per 1,000 PatientsEpilepsy
1.0
3.4
3.5
2.4
Psychiatric
5.7
8.5
1.5
2.9
Other
1.0
1.8
1.9
0.9
Total
2.4
4.3
1.8
1.9
The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing clobazam or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
- Neonatal Sedation and Withdrawal Syndrome [see]
5.9 Neonatal Sedation and Withdrawal SyndromeUse of clobazam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying and feeding difficulties) in the neonate
[see Use in Specific Populations (8.1)]. Monitor neonates exposed to clobazam during pregnancy or labor for signs of sedation and monitor neonates exposed to clobazam during pregnancy for signs of withdrawal; manage these neonates accordingly.
Established Name: | Clobazam Oral Suspension |
Dosage Forms: | Oral Suspension |
Route of Administration: | Oral |
Established Pharmacologic Class of Drug: | Benzodiazepine |
Chemical Name: | 7-Chloro-1-methyl-5-phenyl-1H-1,5 benzodiazepine-2,4( 3H,5H )-dione |
Structural Formula: | 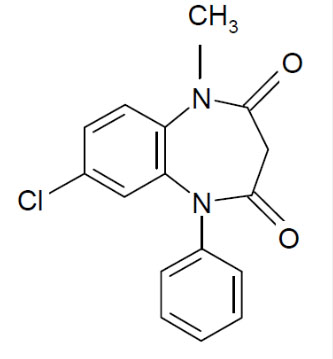 |
Clobazam, USP is a white or almost white, crystalline powder with a slightly bitter taste; is slightly soluble in water, sparingly soluble in ethanol, and freely soluble in methylene chloride. The melting range of clobazam, USP is from 182°C to 185°C. The molecular formula is C16H13O2N2Cl and the molecular weight is 300.7.
Clobazam oral suspension is available for oral administration as an off-white suspension containing clobazam, USP at a concentration of 2.5 mg/mL. Inactive ingredients include berry flavor, citric acid monohydrate, dibasic sodium phosphate dihydrate, magnesium aluminum silicate, maltitol solution, methylparaben, polysorbate 80, propylene glycol, propylparaben, purified water, simethicone emulsion, sucralose, xanthan gum.