Clobetasol Propionate
Clobetasol Propionate Prescribing Information
Clobetasol propionate ointment is super-high potency corticosteroid formulations indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid- responsive dermatoses. Treatment beyond 2 consecutive weeks is not recommended, and the total dosage should not exceed 50 g/week because of the potential for the drug to suppress the hypothalamic-pituitary-adrenal (HPA) axis. Use in pediatric patients under 12 years of age is not recommended.
As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
Apply a thin layer of clobetasol propionate ointment USP, to the affected skin areas twice daily and rub in gently and completely (see INDICATIONS AND USAGE).
Clobetasol propionate ointment USP, is super-high potency topical corticosteroids; therefore, treatment should be limited to 2 consecutive weeks and amounts greater than 50 g/week should not be used.
As with other highly active corticosteroids, therapy should be discontinued when control has been achieved. If no improvement is seen within 2 weeks, reassessment of diagnosis may be necessary.
Clobetasol propionate ointment USP, should not be used with occlusive dressings.
In studies where geriatric patients (65 years of age or older, see PRECAUTIONS) have been treated with clobetasol propionate ointment safety did not differ from that in younger patients; therefore, no dosage adjustment is recommended.
Clobetasol propionate ointment is contraindicated in those patients with a history of hypersensitivity to any of the components of the preparations.
In controlled clinical trials, the most frequent adverse events reported for clobetasol propionate ointment were burning sensation, irritation, and itching in 0.5% of treated patients. Less frequent adverse reactions were stinging, cracking, erythema, folliculitis, numbness of fingers, skin atrophy, and telangiectasia.
Cushing's syndrome has been reported in infants and adults as a result of prolonged use of topical clobetasol propionate formulations.
The following additional local adverse reactions have been reported with topical corticosteroids, and they may occur more frequently with the use of occlusive dressings and higher potency corticosteroids. These reactions are listed in an approximately decreasing order of occurrence: dryness, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, irritation, striae, and miliaria.
Chemically, clobetasol propionate is (11β, 16 β)-21-chloro-9-fluoro-11-hydroxy-16-methyt-17-(1-oxopropoxy)-pregna-1,4-diene-3,20-dione, and it has the following structural formula:
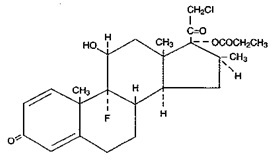
Clobetasol propionate has the empirical formula C25H32CIF05 and a molecular weight of 467. It is a white to cream-colored crystalline powder insoluble in water.
Clobetasol propionate ointment USP, clobetasol propionate 0.5 mg/g in a base of propylene glycol, sorbitan sesquioleate, and white petrolatum.
Like other topical corticosteroids, clobetasol propionate has anti-inflammatory, antipruritic, and vasoconstrictive properties. The mechanism of the anti-inflammatory activity of the topical steroids, in general, is unclear.
However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Occlusive dressing with hydrocortisone for up to 24 hours has not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption.
Studies performed with clobetasol propionate ointment indicate that they are in the super-high range of potency as compared with other topical corticosteroids.