Colesevelam Hydrochloride
Colesevelam Hydrochloride Prescribing Information
Tablets: 625 mg tablets are yellowish oval shaped tablets, and imprinted with  625 on one side and plain on the other side.
625 on one side and plain on the other side.
Colesevelam hydrochloride tablets are contraindicated in patients with:
- Serum TG concentrations >500 mg/dL [see Warnings and Precautions )]
5.1 Hypertriglyceridemia and PancreatitisColesevelam hydrochloride, like other bile acid sequestrants, can increase serum TG concentrations. Hypertriglyceridemia can cause acute pancreatitis.
Colesevelam hydrochloride had effects on serum TG (median increase 5% compared to placebo) in trials of patients with primary hyperlipidemia.
In trials in patients with type 2 diabetes, greater increases in TG levels occurred when colesevelam hydrochloride was used as monotherapy (median increase 9.7% compared to placebo) and when colesevelam hydrochloride was used in combination with pioglitazone (median increase 11% compared to placebo in combination with pioglitazone), sulfonylureas (median increase 18% compared to placebo in combination with sulfonylureas), and insulin (median increase 22% compared to placebo in combination with insulin) [see Adverse Reactions ].
Obtain lipid parameters, including TG levels, before starting colesevelam hydrochloride and periodically thereafter. Colesevelam hydrochloride tablets are contraindicated in patients with TG levels >500 mg/dL or patients with a history of hypertriglyceridemia-induced pancreatitis [see Contraindications ]. Patients with TG levels greater than 300 mg/dL could have greater increases in serum TG levels with colesevelam hydrochloride and may require additional TG monitoring. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting). Discontinue colesevelam hydrochloride if TG levels exceed 500 mg/dL [see Adverse Reactions ].
Obtain lipid parameters, including TG levels, before starting colesevelam hydrochloride and periodically thereafter. Colesevelam hydrochloride tablets are contraindicated in patients with TG levels >500 mg/dL or patients with a history of hypertriglyceridemia-induced pancreatitis [see Contraindications ]. Patients with TG levels greater than 300 mg/dL could have greater increases in serum TG levels with colesevelam hydrochloride and may require additional TG monitoring. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting). Discontinue colesevelam hydrochloride if TG levels exceed 500 mg/dL [see Adverse Reactions ]. Because of its constipating effects, colesevelam hydrochloride is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction. Colesevelam hydrochloride is contraindicated in patients with a history of bowel obstruction [see Contraindications (4)]. Instruct patients to promptly discontinue colesevelam hydrochloride and seek medical attention if severe abdominal pain or severe constipation occurs.
Because of the tablet size, colesevelam hydrochloride tablets can cause dysphagia or esophageal obstruction. For patients with difficulty swallowing tablets, use colesevelam hydrochloride for oral suspension.
The following important adverse reactions are described below and elsewhere in the labeling:
- Hypertriglyceridemia and Pancreatitis [see Warnings and Precautions ]
5.1 Hypertriglyceridemia and PancreatitisColesevelam hydrochloride, like other bile acid sequestrants, can increase serum TG concentrations. Hypertriglyceridemia can cause acute pancreatitis.
Colesevelam hydrochloride had effects on serum TG (median increase 5% compared to placebo) in trials of patients with primary hyperlipidemia.
In trials in patients with type 2 diabetes, greater increases in TG levels occurred when colesevelam hydrochloride was used as monotherapy (median increase 9.7% compared to placebo) and when colesevelam hydrochloride was used in combination with pioglitazone (median increase 11% compared to placebo in combination with pioglitazone), sulfonylureas (median increase 18% compared to placebo in combination with sulfonylureas), and insulin (median increase 22% compared to placebo in combination with insulin) [see Adverse Reactions ].
Obtain lipid parameters, including TG levels, before starting colesevelam hydrochloride and periodically thereafter. Colesevelam hydrochloride tablets are contraindicated in patients with TG levels >500 mg/dL or patients with a history of hypertriglyceridemia-induced pancreatitis [see Contraindications ]. Patients with TG levels greater than 300 mg/dL could have greater increases in serum TG levels with colesevelam hydrochloride and may require additional TG monitoring. Instruct patients to discontinue colesevelam hydrochloride and seek prompt medical attention if the symptoms of acute pancreatitis occur (e.g., severe abdominal pain with or without nausea and vomiting). Discontinue colesevelam hydrochloride if TG levels exceed 500 mg/dL [see Adverse Reactions ]. Because of its constipating effects, colesevelam hydrochloride is not recommended in patients with gastroparesis, other gastrointestinal motility disorders, and in those who have had major gastrointestinal tract surgery and who may be at risk for bowel obstruction. Colesevelam hydrochloride is contraindicated in patients with a history of bowel obstruction [see Contraindications (4)]. Instruct patients to promptly discontinue colesevelam hydrochloride and seek medical attention if severe abdominal pain or severe constipation occurs.
Because of the tablet size, colesevelam hydrochloride tablets can cause dysphagia or esophageal obstruction. For patients with difficulty swallowing tablets, use colesevelam hydrochloride for oral suspension.
- Vitamin K or Fat-Soluble Vitamin Deficiencies [see Warnings and Precautions (]
5.3 Vitamin K or Fat-Soluble Vitamin DeficienciesColesevelam hydrochloride may decrease the absorption of fat-soluble vitamins A, D, E, and K. Patients with a susceptibility to deficiencies of vitamin K (e.g., patients on warfarin, patients with malabsorption syndromes) or other fat-soluble vitamins may be at increased risk when taking colesevelam hydrochloride.
Patients on oral vitamin supplementation should take their vitamins at least 4 hours prior to colesevelam hydrochloride [see Drug Interactions (7.1)].
Colesevelam hydrochloride is a non-absorbed, polymeric, lipid-lowering and glucose-lowering agent for oral administration. Colesevelam hydrochloride is a high-capacity bile acid-binding molecule.
Colesevelam hydrochloride is poly(allylamine hydrochloride) cross-linked with epichlorohydrin and alkylated with 1-bromodecane and (6-bromohexyl)-trimethylammonium bromide. The chemical name (IUPAC) of colesevelam hydrochloride is allylamine polymer with 1-chloro-2,3-epoxypropane, [6-(allylamino)-hexyl]trimethylammonium chloride and N-allyldecylamine, hydrochloride. The chemical structure of colesevelam hydrochloride is represented by the following formula:
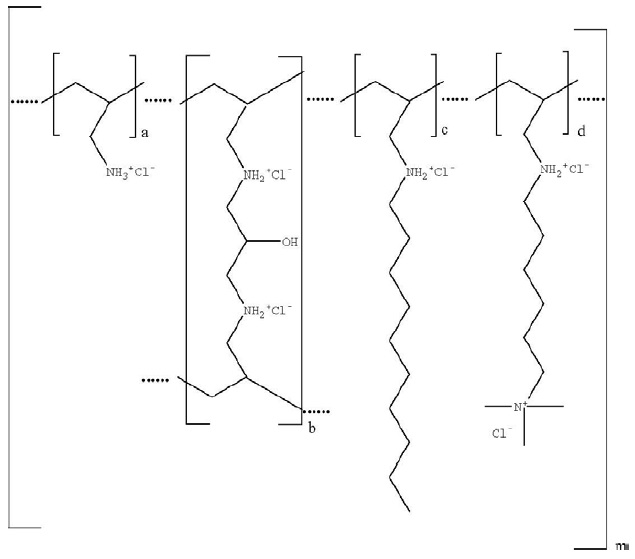
wherein (a) represents allyl amine monomer units that have not been alkylated by either of the 1-bromodecane or (6-bromohexyl)-trimethylammonium bromide alkylating agents or cross-linked by epichlorohydrin; (b) represents allyl amine units that have undergone cross-linking with epichlorohydrin; (c) represents allyl amine units that have been alkylated with a decyl group; (d) represents allyl amine units that have been alkylated with a (6-trimethylammonium) hexyl group, and m represents a number ≥100 to indicate an extended polymer network. A small amount of the amines are dialkylated and are not depicted in the formula above. No regular order of the groups is implied by the structure; cross-linking and alkylation are expected to occur randomly along the polymer chains. A large amount of the amines are protonated. The polymer is depicted in the hydrochloride form; a small amount of the halides are bromide.
Colesevelam hydrochloride is off-white to light yellow powder. Colesevelam hydrochloride is hydrophilic, insoluble in water and any organic solvent.
Colesevelam hydrochloride tablets are yellowish, oval shaped tablet each containing 625 mg colesevelam hydrochloride. In addition, each tablet contains the following inactive ingredients: di-acetylated monoglycerides, hypromellose, iron oxide yellow, magnesium stearate, microcrystalline cellulose and silicon dioxide. The tablets are imprinted using ammonium hydroxide, black iron oxide, polyethylene glycol, propylene glycol and shellac.
Colesevelam hydrochloride tablets 625 mg, are supplied as an yellowish oval shaped tablets imprinted with  625 on one side and plain on the other side. Colesevelam hydrochloride tablets are available as follows:
625 on one side and plain on the other side. Colesevelam hydrochloride tablets are available as follows:
Bottles of 180 NDC 43598-230-18
Store at 20°C to 25°C (68°F to 77°F); [See USP Controlled Room Temperature]. Brief exposure to 40°C (104°F) does not adversely affect the colesevelam hydrochloride tablets. Protect from moisture.