Combipatch (estradiol/norethindrone Acetate Transdermal System)
(Estradiol/Norethindrone Acetate Transdermal System)Combipatch (Estradiol/Norethindrone Acetate Transdermal System) Prescribing Information
CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER, AND PROBABLE DEMENTIA
Estrogen Plus Progestin Therapy
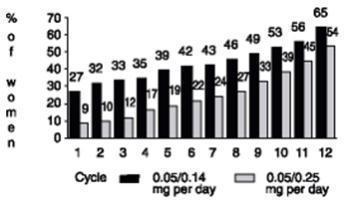
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the
The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogen therapy on blood pressure was not seen.
In women with preexisting hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
Although transdermally administered estrogen therapy avoids first-pass hepatic metabolism, estrogens may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4and T3serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
Estrogens plus progestins may cause some degree of fluid retention. Women with conditions which might be influenced by this factor, such as cardiac or renal impairment warrant careful observation when estrogens plus progestins are prescribed.
Estrogen therapy should be used with caution in women with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For women known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine or porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
Physicians are advised to discuss the content of the
Serum FSH and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Laboratory parameters may be useful in guiding dosage for the treatment of hypoestrogenism due to hypogonadism, castration and primary ovarian failure.
1. Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex; and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III; decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
2. Increased TBG leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4levels (by column or by radioimmunoassay) or T3levels by radioimmunoassay. T3resin uptake is decreased, reflecting the elevated TBG. Free T4and free T3concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
3. Other binding proteins may be elevated in serum (i.e., corticosteroid binding globulin [CBG], SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
4. Increased plasma high density lipoprotein (HDL) and HDL-2 subfraction concentrations, reduced low density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels.
5. Impaired glucose tolerance.
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
NETA was not mutagenic in a battery of
CombiPatch should not be used during pregnancy. (See
CombiPatch should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogens and progestins have been identified in the breast milk of women receiving these drugs. Caution should be exercised when CombiPatch is administered to a nursing woman.
CombiPatch is not indicated in children. Clinical studies have not been conducted in the pediatric population.
There have not been sufficient numbers of geriatric women involved in studies utilizing CombiPatch to determine whether those over 65 years of age differ from younger subjects in their response to CombiPatch.
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age. (See
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo. (See
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
There have not been sufficient numbers of geriatric women involved in studies utilizing CombiPatch to determine whether those over 65 years of age differ from younger subjects in their response to CombiPatch.
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age. (See
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo. (See
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
Estrogen-Alone Therapy
See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
In 2 clinical trials designed to assess the degree of relief of moderate to severe vasomotor symptoms in postmenopausal women (n=332), CombiPatch was administered for 3 28-day cycles in
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. 6Total number of patients with available data is 56. 7Total number of patients with available data is 50. | |||
CombiPatch Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=57 | 0.05/0.25 mg per day2 n=52 | n=51 |
Number of Hot Flushes 3 | -9.35 | -8.95 | -6.2 |
Daily Intensity of Hot Flushes 3,4 | -4.65,6 | -5.05 | -2.87 |
| 1Means were adjusted for imbalance among treatment groups and investigators (least squares mean from ANOVA). 2Represents the milligrams of estradiol/NETA delivered daily by each system. 3Population represents those patients who had baseline and endpoint observations. 4The intensity of hot flushes was evaluated on a scale of 0 to 9 (none=0, mild=1-3, moderate= 4-6, severe=7-9). 5P-value versus placebo = <0.001. | |||
CombiPatch ®Continuous Combined | Placebo | ||
|---|---|---|---|
| Adjusted Mean Change from Baseline1 | 0.05/0.14 mg per day2 n=54 | 0.05/0.25 mg per day2 n=59 | n=53 |
| Number of Hot Flushes3 | -9.35 | -9.55 | -5.5 |
| Daily Intensity of Hot Flushes3,4 | -4.45 | -4.55 | -2.1 |
The use of unopposed estrogen therapy has been associated with an increased risk of endometrial hyperplasia, a possible precursor of endometrial adenocarcinoma. Progestins counter the estrogenic effects by decreasing the number of nuclear estradiol receptors and suppressing epithelial DNA synthesis in endometrial tissue.
Clinical studies indicate that the addition of a progestin to an estrogen regimen at least 12 days per cycle reduces the incidence of endometrial hyperplasia and the potential risk of adenocarcinoma in women with intact uteri. The addition of a progestin to an estrogen regimen has not been shown to interfere with the efficacy of estrogen therapy for its approved indications.
CombiPatch was effective in reducing the incidence of estrogen-induced endometrial hyperplasia after 1 year of therapy in two clinical trials. Nine hundred fifty-five (955) postmenopausal women (with intact uteri) were treated with (i) a continuous regimen of CombiPatch alone (
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous combined regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5One of 39 patients had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Combined | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 123 | 98 | 103 |
Number (%) of Patients with Hyperplasia | 1 (<1%)3 | 1 (1%)3,4 | 39 (38%)5 |
| 1Represents milligrams of estradiol/NETA delivered daily by each system. 2Biopsy after 12 cycles of treatment or hyperplasia before cycle 12. 3Comparison of continuous sequential regimen versus estradiol-only patch was significant (p <0.001). 4This patient had hyperplasia at baseline. 5This patient had hyperplasia in an endometrial polyp. | |||
CombiPatch Continuous Sequential | Vivelle Continuous | ||
|---|---|---|---|
| 0.05/0.14 mg per day1 | 0.05/0.25 mg per day1 | 0.05 mg per day | |
Number of Patients with Biopsies 2 | 117 | 114 | 115 |
Number (%) of Patients with Hyperplasia | 1 (<1%) 3,4 | 1 (<1%)3,5 | 23 (20%) |
With the

The WHI enrolled approximately 27,000 predominantly healthy postmenopausal women in two substudies to assess the risks and benefits of daily oral CE (0.625 mg)-alone or in combination with MPA (2.5 mg) compared to placebo in the prevention of certain chronic diseases. The primary endpoint was the incidence of coronary heart disease (CHD) [defined as nonfatal MI, silent MI and CHD death], with invasive breast cancer as the primary adverse outcome. A “global index” included the earliest occurrence of CHD, invasive breast cancer, stroke, PE, endometrial cancer (only in the CE plus MPA substudy), colorectal cancer, hip fracture, or death due to other cause. These substudies did not evaluate the effects of CE plus MPA or CE-alone on menopausal symptoms.
The WHI estrogen plus progestin substudy was stopped early. According to the predefined stopping rule, after an average follow-up of 5.6 years of treatment, the increased risk of invasive breast cancer and cardiovascular events exceeded the specified benefits included in the “global index.” The absolute excess risk of events included in the “global index” was 19 per 10,000 women-years.
For those outcomes included in the WHI “global index” that reached statistical significance after 5.6 years of follow-up, the absolute excess risks per 10,000 women years in the group treated with CE plus MPA were 7 more CHD events, 8 more strokes, 10 more PEs, and 8 more invasive breast cancers, while the absolute risk reductions per 10,000 women-years were 6 fewer colorectal cancers and 5 fewer hip fractures.
Results of the estrogen plus progestin substudy, which included 16,608 women (average 63 years of age, range 50 to 79 years; 83.9 percent white, 6.8 percent black, 5.4 percent Hispanic, 3.9 percent Other), are presented in Table 7. These results reflect centrally adjudicated data after an average follow-up of 5.6 years.
a Adapted from numerous WHI publications. WHI publications can be viewed at www.nhlbi.nih.gov/whi.b Results are based on centrally adjudicated data.c Nominal confidence intervals (CI) unadjusted for multiple looks and multiple comparisons.d Not included in “global index”.e Includes metastatic and non-metastatic breast cancer, with the exception of in situ breast cancer.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE/MPA vs. Placebo (95% nCIc) | CE/MPA n=8,506 | Placebo n=8,102 |
Absolute Risk per 10,000 Women-Years | |||
| CHD events | 1.23 (0.99–1.53) | 41 | 34 |
Nonfatal MI | 1.28 (1.00–1.63) | 31 | 25 |
CHD death | 1.10 (0.70–1.75) | 8 | 8 |
| All strokes | 1.31 (1.03–1.68) | 33 | 25 |
Ischemic stroke | 1.44 (1.09–1.90) | 26 | 18 |
| Deep vein thrombosis d | 1.95 (1.43–2.67) | 26 | 13 |
| Pulmonary embolism | 2.13 (1.45–3.11) | 18 | 8 |
| Invasive breast cancer e | 1.24 (1.01–1.54) | 41 | 33 |
| Colorectal cancer | 0.61 (0.42–0.87) | 10 | 16 |
| Endometrial cancer d | 0.81 (0.48–1.36) | 6 | 7 |
| Cervical cancer d | 1.44 (0.47–4.42) | 2 | 1 |
| Hip fracture | 0.67 (0.47–0.96) | 11 | 16 |
| Vertebral fractures d | 0.65 (0.46–0.92) | 11 | 17 |
| Lower arm/wrist fractures d | 0.71 (0.59–0.85) | 44 | 62 |
| Total fractures d | 0.76 (0.69–0.83) | 152 | 199 |
| Overall mortality f | 1.00 (0.83–1.19) | 52 | 52 |
| Global Index g | 1.13 (1.02–1.25) | 184 | 165 |
Timing of the initiation of estrogen plus progestin therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen plus progestin substudy stratified by age showed in women 50 to 59 years of age a nonsignificant trend toward reduced risk for overall mortality [hazard ratio (HR) 0.69 (95 percent CI 0.44 to 1.07)].
The WHI estrogen-alone substudy was stopped early because an increased risk of stroke was observed, and it was deemed that no further information would be obtained regarding the risks and benefits of estrogen-alone in predetermined primary endpoints.
Results of the estrogen-alone substudy, which included 10,739 women (average 63 years of age, range 50 to 79 years; 75.3 percent white, 15.1 percent black, 6.1 percent Hispanic, 3.6 percent Other) after an average follow-up of 7.1 years, are presented in Table 8.
a Adapted from numerous WHI publications. WHI publications can be viewed atwww.nhlbi.nih.gov/whi .b Nominal CI unadjusted for multiple looks and multiple comparisons.c Results are based on centrally adjudicated data for an average follow-up of 7.1 years.d Not included in “global index”.e Results are based on an average follow-up of 6.8 years.f All deaths, except from breast or colorectal cancer, definite or probable CHD, PE or cerebrovascular disease.g A subset of the events was combined in a “global index”, defined as the earliest occurrence of CHD events, invasive breast cancer, stroke, pulmonary embolism, colorectal cancer, hip fracture, or death due to other causes. | |||
| Event | Relative Risk CE vs. Placebo (95% nCIb) | CE n=5,310 | Placebo n=5,429 |
Absolute Risk per 10,000 Women-Years | |||
CHD eventsc | 0.95 (0.78–1.16) | 54 | 57 |
Nonfatal MI c | 0.91 (0.73–1.14) | 40 | 43 |
CHD death c | 1.01 (0.71–1.43) | 16 | 16 |
| All strokes c | 1.33 (1.05–1.68) | 45 | 33 |
Ischemic stroke c | 1.55 (1.19–2.01) | 38 | 25 |
| Deep vein thrombosis c,d | 1.47 (1.06–2.06) | 23 | 15 |
| Pulmonary embolism c | 1.37 (0.90–2.07) | 14 | 10 |
| Invasive breast cancer c | 0.80 (0.62–1.04) | 28 | 34 |
| Colorectal cancer e | 1.08 (0.75–1.55) | 17 | 16 |
| Hip fracture c | 0.65 (0.45–0.94) | 12 | 19 |
| Vertebral fractures c,d | 0.64 (0.44–0.93) | 11 | 18 |
| Lower arm/wrist fractures c,d | 0.58 (0.47–0.72) | 35 | 59 |
| Total fractures c,d | 0.71 (0.64–0.80) | 144 | 197 |
| Death due to other causes e,f | 1.08 (0.88–1.32) | 53 | 50 |
| Overall mortality c,d | 1.04 (0.88–1.22) | 79 | 75 |
| Global Index g | 1.02 (0.92–1.13) | 206 | 201 |
For those outcomes included in the WHI “global index” that reached statistical significance, the absolute excess risk per 10,000 women-years in the group treated with CE-alone was 12 more strokes, while the absolute risk reduction per 10,000 women-years was 7 fewer hip fractures.9The absolute excess risk of events included in the “global index” was a nonsignificant 5 events per 10,000 women-years. There was no difference between the groups in terms of all-cause mortality.
No overall difference for primary CHD events (nonfatal MI, silent MI and CHD death) and invasive breast cancer incidence in women receiving CE-alone compared with placebo was reported in final centrally adjudicated results from the estrogen-alone substudy, after an average follow-up of 7.1 years (see Table 8).
Centrally adjudicated results for stroke events from the estrogen-alone substudy, after an average follow-up of 7.1 years, reported no significant difference in distribution of stroke subtype or severity, including fatal strokes, in women receiving CE-alone compared to placebo. Estrogen-alone increased the risk for ischemic stroke, and this excess was present in all subgroups of women examined.10(see Table 8).
Timing of the initiation of estrogen therapy relative to the start of menopause may affect the overall risk benefit profile. The WHI estrogen-alone substudy stratified by age showed in women 50 to 59 years of age, a nonsignificant trend toward reduced risk for CHD [HR 0.63 (95 percent, CI 0.36 to 1.09)] and overall mortality [HR 0.71 (95 percent CI, 0.46 to 1.11)].
The WHIMS estrogen plus progestin ancillary study of WHI enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were 65 to 69 years of age; 35 percent were 70 to 74 years of age; 18 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg) plus MPA (2.5 mg) on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 4 years, the relative risk of probable dementia for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years. Probable dementia as defined in this study included Alzheimer disease (AD), vascular dementia (VaD) and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
The WHIMS estrogen-alone ancillary study of WHI enrolled 2,947 predominantly healthy hysterectomized postmenopausal women 65 to 79 years of age (45 percent were 65 to 69 years of age; 36 percent were 70 to 74 years of age; 19 percent were 75 years of age and older) to evaluate the effects of daily CE (0.625 mg)-alone on the incidence of probable dementia (primary outcome) compared to placebo.
After an average follow-up of 5.2 years, the relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years. Probable dementia as defined in this study included AD, VaD and mixed type (having features of both AD and VaD). The most common classification of probable dementia in the treatment group and the placebo group was AD. Since the ancillary study was conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
When data from the two populations were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Differences between groups became apparent in the first year of treatment. It is unknown whether these findings apply to younger postmenopausal women. (See

See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogen therapy on blood pressure was not seen.
In women with preexisting hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
Although transdermally administered estrogen therapy avoids first-pass hepatic metabolism, estrogens may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4and T3serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
Estrogens plus progestins may cause some degree of fluid retention. Women with conditions which might be influenced by this factor, such as cardiac or renal impairment warrant careful observation when estrogens plus progestins are prescribed.
Estrogen therapy should be used with caution in women with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For women known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine or porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
Physicians are advised to discuss the content of the
Serum FSH and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Laboratory parameters may be useful in guiding dosage for the treatment of hypoestrogenism due to hypogonadism, castration and primary ovarian failure.
1. Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex; and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III; decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
2. Increased TBG leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4levels (by column or by radioimmunoassay) or T3levels by radioimmunoassay. T3resin uptake is decreased, reflecting the elevated TBG. Free T4and free T3concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
3. Other binding proteins may be elevated in serum (i.e., corticosteroid binding globulin [CBG], SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
4. Increased plasma high density lipoprotein (HDL) and HDL-2 subfraction concentrations, reduced low density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels.
5. Impaired glucose tolerance.
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
NETA was not mutagenic in a battery of
CombiPatch should not be used during pregnancy. (See
CombiPatch should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogens and progestins have been identified in the breast milk of women receiving these drugs. Caution should be exercised when CombiPatch is administered to a nursing woman.
CombiPatch is not indicated in children. Clinical studies have not been conducted in the pediatric population.
There have not been sufficient numbers of geriatric women involved in studies utilizing CombiPatch to determine whether those over 65 years of age differ from younger subjects in their response to CombiPatch.
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age. (See
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo. (See
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
There have not been sufficient numbers of geriatric women involved in studies utilizing CombiPatch to determine whether those over 65 years of age differ from younger subjects in their response to CombiPatch.
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age. (See
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo. (See
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
CombiPatch is indicated in a woman with a uterus for:
- Treatment of moderate to severe vasomotor symptoms due to menopause.
- Treatment of moderate to severe symptoms of vulvar and vaginal atrophy due to menopause. When prescribing solely for the treatment of symptoms of vulvar and vaginal atrophy, topical vaginal products should be considered.
- Treatment of hypoestrogenism due to hypogonadism, castration, or primary ovarian failure.
Generally, when estrogen therapy is prescribed for a postmenopausal woman with a uterus, a progestin should be considered to reduce the risk of endometrial cancer. A woman without a uterus generally does not need a progestin. In some cases, however, hysterectomized women with a history of endometriosis may need a progestin. Use of estrogen-alone or in combination with a progestin, should be with the lowest effective dose and for the shortest duration consistent with treatment goals and risks for the individual woman. Postmenopausal women should be reevaluated periodically as clinically appropriate to determine whether treatment is still necessary. Adequate diagnostic measures, such as directed or random endometrial sampling, when indicated, should be undertaken to rule out malignancy in a postmenopausal woman with a uterus with undiagnosed persistent or recurring abnormal genital bleeding.
Patients should be started at the lowest dose. Estrogens with or without progestins should be prescribed at the lowest effective doses and for the shortest duration consistent with treatment goals and risks for the individual woman. The lowest effective dose of CombiPatch has not been determined in clinical trials.
Women not currently using continuous estrogen or combination estrogen plus progestin therapy may start therapy with CombiPatch at any time. However, women currently using continuous estrogen or combination estrogen plus progestin therapy should complete the current cycle of therapy, before initiating CombiPatch therapy.
Women often experience withdrawal bleeding at the completion of the cycle. The first day of this bleeding would be an appropriate time to begin CombiPatch therapy.
Combination estrogen plus progestin regimens are indicated for women with an intact uterus. Two CombiPatch (estradiol/NETA) transdermal delivery systems are available: 0.05 mg estradiol with 0.14 mg NETA per day (9 cm2) and 0.05 mg estradiol with 0.25 mg NETA per day (16 cm2). The lowest effective dose should be used. For all regimens, women should be reevaluated at 3- to 6-month intervals to determine if changes in hormone therapy or if continued hormone therapy is appropriate.
CombiPatch 0.05 mg estradiol/0.14 mg NETA per day (9 cm2) matrix transdermal system is used for continuous uninterrupted treatment applied twice weekly on the lower abdomen. A new system should be applied to the skin every 3 to 4 days (twice weekly) during a 28-day cycle.
Additionally, a dose of 0.05 mg estradiol/0.25 mg NETA (16 cm2 system) is available if a greater progestin dose is desired. Irregular bleeding may occur particularly in the first six months, but generally decreases with time, and often to an amenorrheic state.
CombiPatch can be applied as a sequential regimen in combination with an estradiol-only transdermal delivery system.
In this treatment regimen, a 0.05 mg per day (nominal delivery rate) estradiol transdermal system (Vivelle-Dot®) is worn for the first 14 days of a 28-day cycle, replacing the system every 3 to 4 days (twice weekly) according to product directions.
For the remaining 14 days of the 28-day cycle, CombiPatch 0.05 mg estradiol/0.14 mg NETA per day (9 cm2) transdermal system should be worn continuously on the lower abdomen. The CombiPatch system should be replaced every 3 to 4 days (twice weekly) during this 14-day period in the 28-day cycle.
Additionally, a dose of 0.05 mg estradiol/0.25 mg NETA (16 cm2 system) is available if a greater progestin dose is desired. Women should be advised that monthly withdrawal bleeding often occurs.
CombiPatch should be placed on a smooth (fold-free), clean, dry area of the skin on the lower abdomen.
After opening the pouch, remove 1 side of the protective liner, taking care not to touch the adhesive part of the transdermal delivery system with the fingers. Immediately apply the transdermal delivery system to a smooth (fold-free) area of skin on the lower abdomen. Remove the second side of the protective liner and press the system firmly in place with the hand for at least 10 seconds, making sure there is good contact, especially around the edges.
Care should be taken that the system does not become dislodged during bathing and other activities. If a system should fall off, the same system may be reapplied to another area of the lower abdomen. If necessary, a new transdermal system may be applied, in which case, the original treatment schedule should be continued.
Once in place, the transdermal system should not be exposed to the sun for prolonged periods of time.
Removal of the system should be done carefully and slowly to avoid irritation of the skin. Should any adhesive remain on the skin after removal of the system, allow the area to dry for 15 minutes. Then gently rub the area with an oil-based cream or lotion to remove the adhesive residue.
CombiPatch is contraindicated in women with any of the following conditions:
- Undiagnosed abnormal genital bleeding.
- Known, suspected, or history of breast cancer.
- Known or suspected estrogen-dependent neoplasia.
- Active DVT, PE, or history of these conditions.
- Active arterial thromboembolic disease (for example, stroke and MI), or a history of these conditions.
- Known anaphylactic reaction or angioedema or hypersensitivity with CombiPatch.
- Known liver impairment or disease.
- Known protein C, protein S, or antithrombin deficiency, or other known thrombophilic disorders.
- Known or suspected pregnancy.
See
CARDIOVASCULAR DISORDERS, BREAST CANCER, ENDOMETRIAL CANCER, AND PROBABLE DEMENTIA
Estrogen Plus Progestin Therapy
Estrogen-Alone Therapy
See
An increased risk of PE, DVT, stroke and MI has been reported with estrogen plus progestin therapy. An increased risk of stroke and DVT has been reported with estrogen-alone therapy. Should any of these occur or be suspected, estrogen with or without progestin therapy should be discontinued immediately.
Risk factors for arterial vascular disease (for example, hypertension, diabetes mellitus, tobacco use, hypercholesterolemia, and obesity) and/or venous thromboembolism (VTE) (for example, personal history or family history of VTE, obesity, and systemic lupus erythematosus) should be managed appropriately.
In the WHI estrogen plus progestin substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women in the same age group receiving placebo (33 versus 25 per 10,000 women-years). (See
In the WHI estrogen-alone substudy, a statistically significant increased risk of stroke was reported in women 50 to 79 years of age receiving daily CE (0.625 mg)-alone compared to women in the same age group receiving placebo (45 versus 33 per 10,000 women-years). (See
Subgroup analyses of women 50 to 59 years of age suggest no increased risk of stroke for those women receiving CE (0.625 mg)-alone versus those receiving placebo (18 versus 21 per 10,000 women-years).1
In the WHI estrogen plus progestin substudy, there was a statistically non-significant increased risk of CHD events (defined as nonfatal MI, silent MI, or CHD death) reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to women receiving placebo (41 versus 34 per 10,000 women years).1An increase in relative risk was demonstrated in year 1, and a trend toward decreasing relative risk was reported in years 2 through 5 (See
In the WHI estrogen-alone substudy, no overall effect on CHD events was reported in women receiving estrogen-alone compared to placebo.2(See
Subgroup analyses of women 50 to 59 years of age suggest a statistically nonsignificant reduction in CHD events (CE [0.625 mg]-alone compared to placebo) in women less than 10 years since menopause (8 versus 16 per 10,000 women-years).1
In postmenopausal women with documented heart disease (n=2,763, average 66.7 years of age), in a controlled clinical trial of secondary prevention of cardiovascular disease (Heart and Estrogen/Progestin Replacement Study; HERS), treatment with daily CE (0.625 mg) plus MPA (2.5 mg) demonstrated no cardiovascular benefit. During an average follow-up of 4.1 years, treatment with CE plus MPA did not reduce the overall rate of CHD events in postmenopausal women with established CHD. There were more CHD events in the CE plus MPA-treated group than in the placebo group in year 1, but not during the subsequent years. Two thousand three hundred twenty-one (2,321) women from the original HERS trial agreed to participate in an open-label extension of HERS, HERS II. Average follow-up in HERS II was an additional 2.7 years, for a total of 6.8 years overall. Rates of CHD events were comparable among women in the CE plus MPA group and the placebo group in the HERS, the HERS II, and overall.
In the WHI estrogen plus progestin substudy, a statistically significant 2-fold greater rate of VTE (DVT and PE) was reported in women receiving daily CE (0.625 mg) plus MPA (2.5 mg) compared to placebo (35 versus 17 per 10,000 women-years). Statistically significant increases in risk for both DVT (26 versus 13 per 10,000 women-years) and PE (18 versus 8 per 10,000 women years) were also demonstrated. The increase in VTE risk was demonstrated during the first year and persisted.3(See
In the WHI estrogen-alone substudy, the risk of VTE was reported to be increased for women receiving daily CE (0.625 mg)-alone compared to women receiving placebo (30 versus 22 per 10,000 women-years), although only the increased risk of DVT reached statistical significance (23 versus 15 per 10,000 women years). The increase in VTE risk was demonstrated during the first 2 years.4(See
If feasible, estrogens should be discontinued at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization.
The use of estrogen-alone and estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
All women should receive yearly breast examinations by a healthcare provider and perform monthly breast self-examinations. In addition, mammography examinations should be scheduled based on patient age, risk factors, and prior mammogram results.
An increased risk of endometrial cancer has been reported with the use of unopposed estrogen therapy in a woman with a uterus. The reported endometrial cancer risk among users of unopposed estrogen is about 2 to 12 times greater than in nonusers, and appears dependent on duration of treatment and on estrogen dose. Most studies show no significant increased risk associated with the use of estrogens for less than 1 year. The greatest risk appears associated with prolonged use with increased risks of 15- to 24-fold for 5 to 10 years or more and this risk has been shown to persist for at least 8 to 15 years after estrogen therapy is discontinued.
Clinical surveillance of all women using estrogen-alone or estrogen plus progestin therapy is important. Adequate diagnostic measures, including directed or random endometrial sampling when indicated, should be undertaken to rule out malignancy in postmenopausal women with undiagnosed persistent or recurring abnormal genital bleeding. There is no evidence that the use of natural estrogens results in a different endometrial risk profile than synthetic estrogens of equivalent estrogen dose. Adding a progestin to estrogen therapy has been shown to reduce the risk of endometrial hyperplasia, which may be a precursor to endometrial cancer.
The WHI estrogen plus progestin substudy reported a statistically nonsignificant increased risk of ovarian cancer. After an average follow-up of 5.6 years, the relative risk for ovarian cancer for CE plus MPA versus placebo was 1.58 (95 percent CI 0.77 to 3.24). The absolute risk for CE plus MPA versus placebo was 4 versus 3 cases per 10,000 women-years.7
A meta-analysis of 17 prospective and 35 retrospective epidemiology studies found that women who used hormonal therapy for menopausal symptoms had an increased risk for ovarian cancer. The primary analysis, using case-control comparisons, included 12,110 cancer cases from the 17 prospective studies. The relative risks associated with current use of hormonal therapy was 1.41 (95% confidence interval [CI] 1.32 to 1.50); there was no difference in the risk estimates by duration of the exposure (less than 5 years [median of 3 years]vs. greater than 5 years [median of 10 years] of use before the cancer diagnosis). The relative risk associated with combined current and recent use (discontinued use within 5 years before cancer diagnosis) was 1.37 (95% CI 1.27-1.48), and the elevated risk was significant for both estrogen-alone and estrogen plus progestin products. The exact duration of hormone therapy use associated with an increased risk of ovarian cancer, however, is unknown.
In the WHIMS estrogen plus progestin ancillary substudy of WHI, a population of 4,532 generally healthy postmenopausal women 65 to 79 years of age was randomized to daily CE (0.625 mg) plus MPA (2.5 mg) or placebo.
After an average follow-up of 4 years, 40 women in the CE plus MPA group and 21 women in the placebo group were diagnosed with probable dementia. The relative risk for CE plus MPA versus placebo was 2.05 (95 percent CI, 1.21 to 3.48). The absolute risk of probable dementia for CE plus MPA versus placebo was 45 versus 22 cases per 10,000 women-years.8(See
In the WHIMS estrogen-alone ancillary study of WHI, a population of 2,947 hysterectomized women 65 to 79 years of age was randomized to daily CE (0.625 mg)-alone or placebo. After an average follow-up of 5.2 years, 28 women in the estrogen-alone group and 19 women in the placebo group were diagnosed with probable dementia. The relative risk of probable dementia for CE-alone versus placebo was 1.49 (95 percent CI, 0.83 to 2.66). The absolute risk of probable dementia for CE-alone versus placebo was 37 versus 25 cases per 10,000 women-years.8(See
When data from the 2 populations in the WHIMS estrogen-alone and estrogen plus progestin ancillary studies were pooled as planned in the WHIMS protocol, the reported overall relative risk for probable dementia was 1.76 (95 percent CI, 1.19 to 2.60). Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women.8(See
A 2- to 4-fold increase in the risk of gallbladder disease requiring surgery in postmenopausal women receiving estrogens has been reported.
Estrogen administration may lead to severe hypercalcemia in patients with breast cancer and bone metastases. If hypercalcemia occurs, use of the drug should be stopped and appropriate measures taken to reduce the serum calcium level.
Retinal vascular thrombosis has been reported in women receiving estrogens. Discontinue medication pending examination if there is sudden partial or complete loss of vision, or a sudden onset of proptosis, diplopia, or migraine. If examination reveals papilledema or retinal vascular lesions, estrogens should be permanently discontinued.
Angioedema involving eye/eyelid, face, larynx, pharynx, tongue and extremity (hands, legs, ankles, and fingers) with or without urticaria requiring medical intervention has occurred in the postmarketing experience of using CombiPatch. If angioedema involves the tongue, glottis, or larynx, airway obstruction may occur. Women who develop angioedema anytime during the course of treatment with CombiPatch should not receive it again. Exogenous estrogens may exacerbate symptoms of angioedema in women with hereditary angioedema.
Cases of anaphylactic/anaphylactoid reactions, which developed anytime during the course of CombiPatch treatment and required emergency medical management, have been reported in the postmarketing setting. Involvement of skin (hives, pruritus, swollen lips-tongue-face) and either respiratory tract (respiratory compromise) or gastrointestinal tract (abdominal pain, vomiting) has been noted.
Studies of the addition of a progestin for 10 or more days of a cycle of estrogen administration, or daily with estrogen in a continuous regimen, have reported a lowered incidence of endometrial hyperplasia than would be induced by estrogen treatment alone. Endometrial hyperplasia may be a precursor to endometrial cancer.
There are, however, possible risks that may be associated with the use of progestins with estrogens compared to estrogen-alone regimens. These include an increased risk of breast cancer.
In a small number of case reports, substantial increases in blood pressure have been attributed to idiosyncratic reactions to estrogens. In a large, randomized, placebo-controlled clinical trial, a generalized effect of estrogen therapy on blood pressure was not seen.
In women with preexisting hypertriglyceridemia, estrogen therapy may be associated with elevations of plasma triglycerides leading to pancreatitis. Consider discontinuation of treatment if pancreatitis occurs.
Although transdermally administered estrogen therapy avoids first-pass hepatic metabolism, estrogens may be poorly metabolized in women with impaired liver function. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, caution should be exercised and in the case of recurrence, medication should be discontinued.
Estrogen administration leads to increased thyroid-binding globulin (TBG) levels. Women with normal thyroid function can compensate for the increased TBG by making more thyroid hormone, thus maintaining free T4and T3serum concentrations in the normal range. Women dependent on thyroid hormone replacement therapy who are also receiving estrogens may require increased doses of their thyroid replacement therapy. These women should have their thyroid function monitored in order to maintain their free thyroid hormone levels in an acceptable range.
Estrogens plus progestins may cause some degree of fluid retention. Women with conditions which might be influenced by this factor, such as cardiac or renal impairment warrant careful observation when estrogens plus progestins are prescribed.
Estrogen therapy should be used with caution in women with hypoparathyroidism as estrogen-induced hypocalcemia may occur.
A few cases of malignant transformation of residual endometrial implants have been reported in women treated post-hysterectomy with estrogen-alone therapy. For women known to have residual endometriosis post-hysterectomy, the addition of progestin should be considered.
Estrogen therapy may cause an exacerbation of asthma, diabetes mellitus, epilepsy, migraine or porphyria, systemic lupus erythematosus, and hepatic hemangiomas and should be used with caution in women with these conditions.
Physicians are advised to discuss the content of the
Serum FSH and estradiol levels have not been shown to be useful in the management of moderate to severe vasomotor symptoms and moderate to severe symptoms of vulvar and vaginal atrophy.
Laboratory parameters may be useful in guiding dosage for the treatment of hypoestrogenism due to hypogonadism, castration and primary ovarian failure.
1. Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, II-VII-X complex; and beta-thromboglobulin; decreased levels of anti-factor Xa and antithrombin III; decreased antithrombin III activity; increased levels of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
2. Increased TBG leading to increased circulating total thyroid hormone, as measured by protein-bound iodine (PBI), T4levels (by column or by radioimmunoassay) or T3levels by radioimmunoassay. T3resin uptake is decreased, reflecting the elevated TBG. Free T4and free T3concentrations are unaltered. Women on thyroid replacement therapy may require higher doses of thyroid hormone.
3. Other binding proteins may be elevated in serum (i.e., corticosteroid binding globulin [CBG], SHBG), leading to increased circulating corticosteroids and sex steroids, respectively. Free hormone concentrations, such as testosterone and estradiol, may be decreased. Other plasma proteins may be increased (angiotensinogen/renin substrate, alpha-1-antitrypsin, ceruloplasmin).
4. Increased plasma high density lipoprotein (HDL) and HDL-2 subfraction concentrations, reduced low density lipoprotein (LDL) cholesterol concentration, increased triglycerides levels.
5. Impaired glucose tolerance.
Long-term continuous administration of natural and synthetic estrogens in certain animal species increases the frequency of carcinomas of the breast, uterus, cervix, vagina, testis, and liver.
NETA was not mutagenic in a battery of
CombiPatch should not be used during pregnancy. (See
CombiPatch should not be used during lactation. Estrogen administration to nursing women has been shown to decrease the quantity and quality of the breast milk. Detectable amounts of estrogens and progestins have been identified in the breast milk of women receiving these drugs. Caution should be exercised when CombiPatch is administered to a nursing woman.
CombiPatch is not indicated in children. Clinical studies have not been conducted in the pediatric population.
There have not been sufficient numbers of geriatric women involved in studies utilizing CombiPatch to determine whether those over 65 years of age differ from younger subjects in their response to CombiPatch.
In the WHI estrogen plus progestin substudy (daily CE [0.625 mg] plus MPA [2.5 mg] versus placebo), there was a higher relative risk of nonfatal stroke and invasive breast cancer in women greater than 65 years of age. (See
In the WHI estrogen-alone substudy (daily CE [0.625 mg]-alone versus placebo), there was a higher relative risk of stroke in women greater than 65 years of age. (See
In the WHIMS ancillary studies of postmenopausal women 65 to 79 years of age, there was an increased risk of developing probable dementia in women receiving estrogen plus progestin or estrogen-alone when compared to placebo. (See
Since both ancillary studies were conducted in women 65 to 79 years of age, it is unknown whether these findings apply to younger postmenopausal women. (See
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
| VASOMOTOR SYMPTOM STUDIES | |||
CombiPatch 0.05/0.14 mg per day† | CombiPatch 0.05/0.25 mg per day† | Placebo | |
| n=113 | n=112 | n=107 | |
| Body as a Whole | 46% | 48% | 41% |
| Abdominal Pain | 7% | 6% | 4% |
| Accidental Injury | 4% | 5% | 8% |
| Asthenia | 8% | 12% | 4% |
| Back Pain | 11% | 9% | 5% |
| Flu Syndrome | 9% | 5% | 7% |
| Headache | 18% | 20% | 20% |
| Pain | 6% | 4% | 9% |
| Digestive | 19% | 23% | 24% |
| Diarrhea | 4% | 5% | 7% |
| Dyspepsia | 1% | 5% | 5% |
| Flatulence | 4% | 5% | 4% |
| Nausea | 11% | 8% | 7% |
| Nervous | 16% | 28% | 28% |
| Depression | 3% | 5% | 9% |
| Insomnia | 3% | 6% | 7% |
| Nervousness | 3% | 5% | 1% |
| Respiratory | 24% | 38% | 26% |
| Pharyngitis | 4% | 10% | 2% |
| Respiratory Disorder | 7% | 12% | 7% |
| Rhinitis | 7% | 13% | 9% |
| Sinusitis | 4% | 9% | 9% |
| Skin and Appendages | 8% | 17% | 16% |
| Application Site Reaction* | 2% | 6% | 4% |
| Urogenital | 54% | 63% | 28% |
| Breast Pain | 25% | 31% | 7% |
| Dysmenorrhea | 20% | 21% | 5% |
| Leukorrhea | 5% | 5% | 3% |
| Menstrual Disorder | 6% | 12% | 2% |
| Papanicolaou Smear Suspicious | 8% | 4% | 5% |
| Vaginitis | 6% | 13% | 5% |
| †Represents milligrams of estradiol/NETA delivered daily by each system. | |||
| *Application site reactions includes localized bleeding, bruising, burning, discomfort, dryness, eczema, edema, erythema, inflammation, irritation, pain, papules, paresthesia, pruritus, rash, skin discoloration, skin pigmentation, swelling, urticaria, and vesicles. | |||
| ENDOMETRIAL HYPERPLASIA STUDIES | |||
CombiPatch 0.05/0.14 mg per day† n=325 | CombiPatch 0.05/0.25 mg per day† n=312 | Vivelle 0.05 mg per day n=318 | |
| Body as a Whole | 61% | 60% | 59% |
| Abdominal Pain | 12% | 14% | 16% |
| Accidental Injury | 10% | 11% | 8% |
| Asthenia | 10% | 13% | 11% |
| Back Pain | 15% | 14% | 13% |
| Flu Syndrome | 14% | 10% | 7% |
| Headache | 25% | 17% | 21% |
| Infection | 5% | 3% | 3% |
| Pain | 19% | 15% | 13% |
| Digestive | 42% | 32% | 31% |
| Constipation | 2% | 5% | 3% |
| Diarrhea | 14% | 9% | 7% |
| Dyspepsia | 8% | 6% | 5% |
| Flatulence | 7% | 5% | 6% |
| Nausea | 8% | 12% | 11% |
| Tooth Disorder | 6% | 4% | 1% |
| Metabolic and Nutritional Disorders | 12% | 13% | 11% |
| Peripheral Edema | 6% | 6% | 5% |
| Musculoskeletal | 17% | 17% | 15% |
| Arthralgia | 6% | 6% | 5% |
| Nervous | 33% | 30% | 28% |
| Depression | 8% | 9% | 8% |
| Dizziness | 6% | 7% | 5% |
| Insomnia | 8% | 6% | 4% |
| Nervousness | 5% | 6% | 3% |
| Respiratory | 45% | 43% | 40% |
| Bronchitis | 5% | 3% | 4% |
| Pharyngitis | 9% | 9% | 8% |
| Respiratory Disorder | 13% | 9% | 13% |
| Rhinitis | 19% | 22% | 17% |
| Sinusitis | 10% | 12% | 12% |
| Skin and Appendages | 38% | 37% | 31% |
| Acne | 4% | 5% | 4% |
| Application Site Reaction* | 20% | 23% | 17% |
| Rash | 6% | 5% | 3% |
| Urogenital | 71% | 79% | 74% |
| Breast Enlargement | 2% | 7% | 2% |
| Breast Pain | 34% | 48% | 40% |
| Dysmenorrhea | 30% | 31% | 19% |
| Leukorrhea | 10% | 8% | 9% |
| Menorrhagia | 2% | 5% | 9% |
| Menstrual Disorder | 17% | 19% | 14% |
| Vaginal Hemorrhage | 3% | 6% | 12% |
| Vaginitis | 9% | 13% | 13% |
| †Represents milligrams of estradiol/NETA delivered daily by each system. | |||
| *Application site reactions includes localized bleeding, bruising, burning, discomfort, dryness, eczema, edema, erythema, inflammation, irritation, pain, papules, paresthesia, pruritus, rash, skin discoloration, skin pigmentation, swelling, urticaria, and vesicles. | |||
Postmarketing Experience
The following additional adverse reactions have been identified during post-approval use of CombiPatch. Because these reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Genitourinary System
Endometrial hyperplasia, endocervical polyp, uterine leiomyomata, fallopian tube cyst, uterine spasms.
Breast
Breast cancer.
Cardiovascular
Hypertension, varicose veins.
Gastrointestinal
Jaundice cholestatic, cholelithiasis, gall bladder disorder, transaminases increased.
Skin
Skin discoloration.
Central Nervous System
Affect lability, libido disorder, migraine, vertigo, paresthesia.
Miscellaneous
Angioedema, hypersensitivity, weight increased.
No drug interaction studies have been conducted with CombiPatch.
Averaging across 6 clinical trials lasting 3 months to 1 year, of 1,287 patients treated, CombiPatch transdermal systems completely adhered to the skin nearly 90 percent of the time over the 3- to 4-day wear period. Less than 2 percent of the patients required reapplication or replacement of systems due to lifting or detachment. Two patients (0.2 percent) discontinued therapy during clinical trials due to adhesion failure.