Conray Prescribing Information
Conray is indicated for use in excretory urography, cerebral angiography, peripheral arteriography, venography, arthrography, direct cholangiography, endoscopic retrograde cholangiopancreatography, contrast enhancement of computed tomographic brain images, cranial computerized angiotomography, intravenous digital subtraction angiography and arterial digital subtraction angiography.
Conray may also be used for enhancement of computed tomographic scans performed for detection and evaluation of lesions in the liver, pancreas, kidneys, abdominal aorta, mediastinum, abdominal cavity and retroperitoneal space. Continuous or multiple scans separated by intervals of 1 to 3 seconds during the first 30 to 90 seconds post-injection of the contrast medium (dynamic CT scanning) may provide enhancement of diagnostic significance, and may be of benefit in establishing diagnoses of certain lesions in these sites with greater assurance than is possible with CT alone, and in supplying additional features of the lesions. In other cases, the contrast agent may allow visualization of lesions not seen with CT alone, or may help to define suspicious lesions seen with unenhanced CT (see
Following intravascular injection, Conray is rapidly transported through the circulatory system to the kidneys and is excreted unchanged in the urine by glomerular filtration. The pharmacokinetics of intravascularly administered radiopaque contrast media are usually best described by a two compartment model with a rapid alpha phase for drug distribution and a slower beta phase for drug elimination. In patients with normal renal function, the alpha and beta half-lives of Conray were approximately 10 and 90 minutes, respectively.
Angiography may be performed following intravascular injection which will permit visualization until significant hemodilution occurs.
Renal accumulation is sufficiently rapid that maximum radiographic density in the calyces and pelves occurs, in most instances, about 3 to 8 minutes after injection. In patients with impaired renal function, diagnostic opacification frequently is achieved only after prolonged periods.
Injectable iodinated contrast agents are excreted either through the kidneys or through the liver. These two excretory pathways are not mutually exclusive, but the main route of excretion seems to be related to the affinity of the contrast medium for serum albumin. Iothalamate salts are poorly bound to serum albumin, and are excreted mainly through the kidneys.
The liver and small intestine provide the major alternate route of excretion. In patients with severe renal impairment, the excretion of this contrast medium through the gallbladder and into the small intestine sharply increases.
Iothalamate salts cross the placental barrier in humans and are excreted unchanged in human milk.
The biliary system, pancreatic duct or joint spaces may be visualized by the direct injection of contrast medium into the region to be studied.
When used for contrast enhancement in computed tomographic brain scanning, the degree of enhancement is directly related to the amount of iodine administered. Rapid injection of the entire dose yields peak blood iodine concentrations immediately following the injection, which fall rapidly over the next five to ten minutes. This can be accounted for by the dilution in the vascular and extracellular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached by about ten minutes; thereafter, the fall becomes exponential. Maximum contrast enhancement frequently occurs after peak blood iodine levels are reached. The delay in maximum contrast enhancement can range from five to forty minutes, depending on the peak iodine levels achieved and the cell type of the lesion. This lag suggests that the contrast enhancement of the image is at least in part dependent on the accumulation of iodine within the lesion and outside the blood pool.
In brain scanning, the contrast medium (Conray) does not accumulate in normal brain tissue due to the presence of the “blood brain barrier.” The increase in x-ray absorption in the normal brain is due to the presence of the contrast agent within the blood pool. A break in the blood brain barrier, such as occurs in malignant tumors of the brain, allows accumulation of contrast medium within the interstitial tumor tissue; adjacent normal brain tissue does not contain the contrast medium.
The image enhancement of non-tumoral lesions, such as arteriovenous malformations and aneurysms, is dependent on the iodine content of the circulating blood pool.
When used for cranial computerized angiotomography, rapid bolus injection and/or infusion combined with rapid CT scanning will provide clear delineation of the cerebral vessels.
In non-neural tissues (during CT of the body), Conray diffuses rapidly from the vascular to the extra-vascular space. Increase in x-ray absorption is related to blood flow, concentration of the contrast medium and extraction of the contrast medium by interstitial tissue, since no barrier exists; contrast enhancement is thus due to the relative differences in extra-vascular diffusion between normal and abnormal tissue, a situation quite different than that in the brain.
The pharmacokinetics of Conray in normal and abnormal tissues has been shown to be variable.
Enhancement of CT with Conray may be of benefit in establishing diagnoses of certain lesions in some sites with greater assurance than is possible with unenhanced CT and in supplying additional features of the lesions. In other cases, the contrast medium may allow visualization of lesions not seen with CT alone or may help to define suspicious lesions seen with unenhanced CT.
Contrast enhancement appears to be greatest within the 30 to 90 seconds after bolus administration of the contrast agent, and after intra-arterial, rather than intravenous, administration. Therefore, the use of a continuous scanning technique (a series of 2 to 3 second scans beginning at the injection - dynamic CT scanning) may improve enhancement and diagnostic assessment of tumors and other lesions, such as an abscess, occasionally revealing more extensive disease. A cyst, or similar non-vascularized lesion, may be distinguished from vascularized solid lesions by comparing enhanced and unenhanced scans; non-vascularized lesions show no change in CT number, whereas vascularized lesions would show an increase. The latter might be benign, malignant or normal, but it is unlikely that it would be a cyst, hematoma, or other non-vascularized lesion.
Because
It is advisable that Conray be at or close to body temperature when injected.
The patient should be instructed to omit the meal that precedes the examination. Appropriate premedication, which may include a barbiturate, tranquilizer or analgesic drug, may be administered prior to the examination.
A preliminary film is recommended to check the position of the patient and the x-ray exposure factors.
If a minor reaction occurs during administration, the injection should be slowed or stopped until the reaction has subsided. If a major reaction occurs, the injection should be discontinued immediately.
Under no circumstances should either corticosteroids or antihistamines be mixed in the same syringe with the contrast medium because of a potential for chemical incompatibility.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration.
Refer to PRECAUTIONS, General, concerning hypersensitivity. Conray should not be used for myelography. Arthrography should not be performed if infection is present in or near the joint. Percutaneous transhepatic cholangiography is contraindicated in patients with coagulation defects and prolonged prothrombin times. Endoscopic retrograde cholangiopancreatography is contraindicated during an acute attack of pancreatitis or during severe clinically evident cholangitis and in patients in whom endoscopy is prohibited.
Adverse reactions to injectable contrast media fall into two categories: chemotoxic reactions and idiosyncratic reactions.
Chemotoxic reactions result from the physio-chemical properties of the contrast media, the dose and speed of injection. All hemodynamic disturbances and injuries to organs or vessels perfused by the contrast medium are included in this category.
Idiosyncratic reactions include all other reactions. They occur more frequently in patients 20 to 40 years old. Idiosyncratic reactions may or may not be dependent on the amount of dose injected, the speed of injection, the mode of injection and the radiographic procedure. Idiosyncratic reactions are subdivided into minor, intermediate and severe. The minor reactions are self-limited and of short duration; the severe reactions are life-threatening and treatment is urgent and mandatory.
Fatalities have been reported following the administration of iodine-containing contrast agents. Based upon clinical literature, the incidence of death is reported to range from one in 10,000 (0.01 percent) to less than one in 100,000 (0.001 percent).
The following adverse reactions have been observed in conjunction with the use of iodine-containing contrast agents.
Conray is a sterile aqueous solution intended for use as a diagnostic radiopaque medium. Conray contains 60% w/v iothalamate meglumine, which is 1-deoxy-1-(methylamino)-D-glucitol 5-acetamido-2,4,6 triiodo-N-methylisophthalamate (salt), and has the following structural formula:
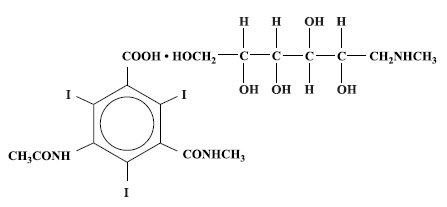
Each milliliter contains 600 mg of iothalamate meglumine, 0.09 mg edetate calcium disodium as a stabilizer and 0.125 mg of monobasic sodium phosphate as a buffer. The solution provides 28.2% (282 mg/mL) organically bound iodine. Conray has an osmolarity of approximately 1000 mOsmol per liter, an osmolality of approximately 1400 mOsmol per kilogram and is, therefore, hypertonic under conditions of use. The viscosity (cps) is approximately 6 at 25°C and 4 at 37°C. The pH is 6.5 to 7.7.
Conray is a clear solution containing no undissolved solids. Crystallization does not occur at normal room temperatures. It is supplied in containers from which the air has been displaced by nitrogen.