Cromolyn Sodium
Cromolyn Sodium Prescribing Information
Cromolyn Sodium Ophthalmic Solution USP, 4% is indicated in the treatment of vernal keratoconjunctivitis, vernal conjunctivitis, and vernal keratitis.
The dose is 1 or 2 drops in each eye 4 to 6 times a day at regular intervals. One drop contains approximately 1.6 mg cromolyn sodium.
Patients should be advised that the effect of cromolyn sodium ophthalmic solution therapy is dependent upon its administration at regular intervals, as directed.
Symptomatic response to therapy (decreased itching, tearing, redness, and discharge) is usually evident within a few days, but longer treatment for up to six weeks is sometimes required. Once symptomatic improvement has been established, therapy should be continued for as long as needed to sustain improvement.
If required, corticosteroids may be used concomitantly with Cromolyn Sodium Ophthalmic Solution USP, 4%.
Cromolyn Sodium Ophthalmic Solution USP, 4% is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium or to any of the other ingredients.
The most frequently reported adverse reaction attributed to the use of cromolyn sodium ophthalmic solution, on the basis of reoccurrence following readministration, is transient ocular stinging or burning upon instillation.
The following adverse reactions have been reported as infrequent events. It is unclear whether they are attributable to the drug:
Conjunctival injection; watery eyes; itchy eyes; dryness around the eye; puffy eyes; eye irritation; and styes.
Immediate hypersensitivity reactions have been reported rarely and include dyspnea, edema and rash.
Cromolyn Sodium Ophthalmic Solution USP, 4% is a clear, colorless, sterile solution intended for topical ophthalmic use.
Cromolyn sodium is represented by the following structural formula:
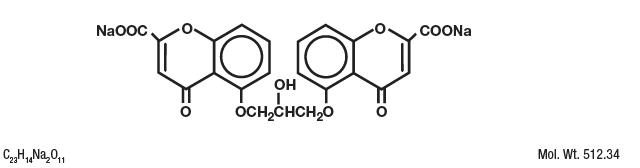
Chemical Name: Disodium 5-5'-[(2-hydroxytrimethylene)dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]
Pharmacologic Category: Mast cell stabilizer.
Another activity demonstrated
Cromolyn sodium has no intrinsic vasoconstrictor, antihistaminic or anti-inflammatory activity.
Cromolyn sodium is poorly absorbed. When multiple doses of cromolyn sodium ophthalmic solution are instilled into normal rabbit eyes, less than 0.07% of the administered dose of cromolyn sodium is absorbed into the systemic circulation (presumably by way of the eye, nasal passages, buccal cavity and gastrointestinal tract). Trace amounts (less than 0.01%) of the cromolyn sodium dose penetrate into the aqueous humor and clearance from this chamber is virtually complete within 24 hours after treatment is stopped.
In normal volunteers, analysis of drug excretion indicates that approximately 0.03% of cromolyn sodium is absorbed following administration to the eye.