Cromolyn Sodium Oral Solution (concentrate)
Cromolyn Sodium Oral Solution (Concentrate) Prescribing Information
Cromolyn Sodium Oral Solution (Concentrate) is indicated in the management of patients with mastocytosis. Use of this product has been associated with improvement in diarrhea, flushing, headaches, vomiting, urticaria, abdominal pain, nausea, and itching in some patients.
NOT FOR INHALATION OR INJECTION. SEE DIRECTIONS FOR USE.
The usual starting dose is as follows:
If satisfactory control of symptoms is not achieved within two to three weeks, the dosage may be increased but should not exceed 40 mg/kg/day. Patients should be advised that the effect of Cromolyn Sodium Oral Solution (Concentrate) therapy is dependent upon its administration at regular intervals, as directed.
1. Break open ampule(s) and squeeze liquid contents of ampule(s) into a glass of water.
2. Stir solution.
3. Drink all of the liquid.
Cromolyn Sodium Oral Solution (Concentrate) is contraindicated in those patients who have shown hypersensitivity to cromolyn sodium.
Most of the adverse events reported in mastocytosis patients have been transient and could represent symptoms of the disease. The most frequently reported adverse events in mastocytosis patients who have received Cromolyn Sodium Oral Solution (Concentrate) during clinical studies were headache and diarrhea, each of which occurred in 4 of the 87 patients. Pruritus, nausea, and myalgia were each reported in 3 patients and abdominal pain, rash, and irritability in 2 patients each. One report of malaise was also recorded.
Other less commonly reported events (the majority representing only a single report) include the following:
| Skin: | pruritus, rash, urticaria/angioedema, erythema/ burning, photosensitivity |
| Musculoskeletal: | arthralgia, myalgia, stiffness/weakness of legs |
| Neurologic: | headache, dizziness, hypoesthesia, paresthesia, migraine, convulsions, flushing |
| Psychiatric: | psychosis, anxiety, depression, hallucinations, behavior change, insomnia, nervousness |
| Heart Rate: | tachycardia, premature ventricular contractions (PVCs), palpitations |
| Respiratory: | pharyngitis, dyspnea |
| Miscellaneous: | fatigue, edema, unpleasant taste, chest pain, postprandial lightheadedness and lethargy, dysuria, urinary frequency, purpura, hepatic function test abnormal, polycythemia, neutropenia, pancytopenia, tinnitus, lupus erythematosus (LE) syndrome |
Each 5 mL ampule of Cromolyn Sodium Oral Solution (Concentrate) contains 100 mg cromolyn sodium, USP, in purified water. Cromolyn sodium is a hygroscopic, white powder having little odor. It may leave a slightly bitter aftertaste. Cromolyn Sodium Oral Solution (Concentrate) is clear, colorless, and sterile. It is intended for oral use.
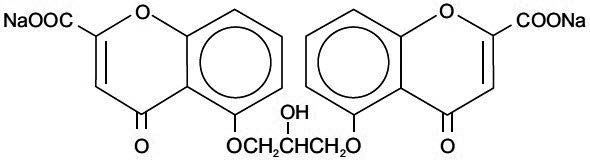
Chemically, cromolyn sodium is disodium 5,5’-[(2-hydroxy-trimethylene) dioxy]bis[4-oxo-4H-1-benzopyran-2-carboxylate]. The empirical formula is C
23H
14Na
2O
11; the molecular weight is 512.34. Its chemical structure is:
Pharmacologic Category: Mast cell stabilizer
Therapeutic Category: Antiallergic
Cromolyn sodium has no intrinsic vasoconstrictor, antihistamine, or glucocorticoid activity.
Cromolyn sodium is poorly absorbed from the gastrointestinal tract. No more than 1% of an administered dose is absorbed by humans after oral administration, the remainder being excreted in the feces. Very little absorption of cromolyn sodium was seen after oral administration of 500 mg by mouth to each of 12 volunteers. From 0.28 to 0.50% of the administered dose was recovered in the first 24 hours of urinary excretion in 3 subjects. The mean urinary excretion of an administered dose over 24 hours in the remaining 9 subjects was 0.45%.