Cyproheptadine Hydrochloride
Cyproheptadine Hydrochloride Prescribing Information
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods.
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema.
Amelioration of allergic reactions to blood or plasma.
Cold urticaria
Dermatographism
As therapy for anaphylactic reactions
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Each tablet contains 4 mg of cyproheptadine hydrochloride.
This drug should
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Hypersensitivity to cyproheptadine and other drugs of similar chemical structure.
Monoamine oxidase inhibitor therapy (see
Angle-closure glaucoma
Stenosing peptic ulcer
Symptomatic prostatic hypertrophy
Bladder neck obstruction
Pyloroduodenal obstruction
Elderly, debilitated patients
Adverse reactions which have been reported with the use of antihistamines are as follows:
MAO inhibitors prolong and intensify the anticholinergic effects of antihistamines.
Antihistamines may have additive effects with alcohol and other CNS depressants, e.g., hypnotics, sedatives, tranquilizers, antianxiety agents.
Cyproheptadine hydrochloride is an antihistaminic and antiserotonergic agent.
Cyproheptadine hydrochloride, USP is a white to slightly yellowish crystalline solid, with a molecular weight of 350.89, which is soluble in water, freely soluble in methanol, sparingly soluble in ethanol, soluble in chloroform, and practically insoluble in ether. It is the sesquihydrate of 4-(5
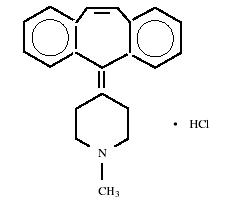
C21H21N•HCl
M.W. 350.89
Cyproheptadine hydrochloride, USP is available for oral administration in 4 mg tablets. Inactive ingredients include: lactose monohydrate, magnesium stearate, microcrystalline cellulose, and pregelatinized starch.