Dabigatran Etexilate Prescribing Information
If possible, discontinue dabigatran etexilate capsules in adults1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required
For pediatric patients, discontinue dabigatran etexilate capsules 24 hours before an elective surgery (eGFR > 80 mL/min/1.73m2) or 2 days before an elective surgery (eGFR 50 to 80 mL/min/1.73m2). Pediatric patients with an eGFR <50 mL/min/1.73m2have not been studied, avoid use of dabigatran etexilate capsules in these patients.
If surgery cannot be delayed, there is an increased risk of bleeding [see
For adult and pediatric patients currently receiving a parenteral anticoagulant, start dabigatran etexilate capsules 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (e.g., intravenous unfractionated heparin).
For adult patients currently taking dabigatran etexilate capsules wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of dabigatran etexilate capsules before initiating treatment with a parenteral anticoagulant
For pediatric patients currently taking dabigatran etexilate, wait 12 hours after the last dose before switching to a parenteral anticoagulant.
If possible, discontinue dabigatran etexilate capsules in adults1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required
For pediatric patients, discontinue dabigatran etexilate capsules 24 hours before an elective surgery (eGFR > 80 mL/min/1.73m2) or 2 days before an elective surgery (eGFR 50 to 80 mL/min/1.73m2). Pediatric patients with an eGFR <50 mL/min/1.73m2have not been studied, avoid use of dabigatran etexilate capsules in these patients.
If surgery cannot be delayed, there is an increased risk of bleeding [see
Premature discontinuation of any oral anticoagulant, including dabigatran etexilate, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. If dabigatran etexilate capsules are discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant and restart dabigatran etexilate capsules as soon as medically appropriate
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran etexilate and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran etexilate and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran etexilate and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
- Non-valvular Atrial Fibrillation in Adult Patients
For patients with CrCl >30 mL/min: 150 mg orally, twice daily (
)2.2 Recommended Dabigatran Etexilate Capsules Dosage for AdultsIndicationDosageReduction in Risk of Stroke and Systemic Embolism in Non-valvular AFCrCl >30 mL/min: 150 mg twice daily CrCl 15 to 30 mL/min: 75 mg twice daily CrCl <15 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl 30 to 50 mL/min with concomitant use of P-gp inhibitors: Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. CrCl <30 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Treatment of DVT and PECrCl >30 mL/min: 150 mg twice daily Reduction in the Risk of Recurrence of DVT and PECrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Prophylaxis of DVT and PE Following Hip Replacement SurgeryCrCl >30 mL/min: 110 mg for first day, then 220 mg once daily CrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors : Avoid co-administration Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation in Adult PatientsFor patients with creatinine clearance (CrCl) >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15 to 30 mL/min), the recommended dosage of dabigatran etexilate capsules is 75 mg twice daily
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.Treatment of Deep Venous Thrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Reduction in the Risk of Recurrence of Deep VenousThrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily after previous treatment. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients Following Hip Replacement SurgeryFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 110 mg taken orally 1 to 4 hours after surgery and after hemostasis has been achieved, then 220 mg taken once daily for 28 to 35 days. If dabigatran etexilate is not started on the day of surgery, after hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Dosage and Administration (2.4), Use in Specific Populations (8.6) and Clinical Pharmacology (12.2,12.3)].For patients with CrCl 15 to 30 mL/min: 75 mg orally, twice daily (
)2.2 Recommended Dabigatran Etexilate Capsules Dosage for AdultsIndicationDosageReduction in Risk of Stroke and Systemic Embolism in Non-valvular AFCrCl >30 mL/min: 150 mg twice daily CrCl 15 to 30 mL/min: 75 mg twice daily CrCl <15 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl 30 to 50 mL/min with concomitant use of P-gp inhibitors: Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. CrCl <30 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Treatment of DVT and PECrCl >30 mL/min: 150 mg twice daily Reduction in the Risk of Recurrence of DVT and PECrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Prophylaxis of DVT and PE Following Hip Replacement SurgeryCrCl >30 mL/min: 110 mg for first day, then 220 mg once daily CrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors : Avoid co-administration Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation in Adult PatientsFor patients with creatinine clearance (CrCl) >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15 to 30 mL/min), the recommended dosage of dabigatran etexilate capsules is 75 mg twice daily
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.Treatment of Deep Venous Thrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Reduction in the Risk of Recurrence of Deep VenousThrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily after previous treatment. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients Following Hip Replacement SurgeryFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 110 mg taken orally 1 to 4 hours after surgery and after hemostasis has been achieved, then 220 mg taken once daily for 28 to 35 days. If dabigatran etexilate is not started on the day of surgery, after hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Dosage and Administration (2.4), Use in Specific Populations (8.6) and Clinical Pharmacology (12.2,12.3)].
- Treatment of DVT and PE:in Adult Patients
- For patients with CrCl >30 mL/min: 150 mg orally, twice daily after 5 to 10 days of parenteral anticoagulation ()
2.2 Recommended Dabigatran Etexilate Capsules Dosage for AdultsIndicationDosageReduction in Risk of Stroke and Systemic Embolism in Non-valvular AFCrCl >30 mL/min: 150 mg twice daily CrCl 15 to 30 mL/min: 75 mg twice daily CrCl <15 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl 30 to 50 mL/min with concomitant use of P-gp inhibitors: Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. CrCl <30 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Treatment of DVT and PECrCl >30 mL/min: 150 mg twice daily Reduction in the Risk of Recurrence of DVT and PECrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Prophylaxis of DVT and PE Following Hip Replacement SurgeryCrCl >30 mL/min: 110 mg for first day, then 220 mg once daily CrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors : Avoid co-administration Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation in Adult PatientsFor patients with creatinine clearance (CrCl) >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15 to 30 mL/min), the recommended dosage of dabigatran etexilate capsules is 75 mg twice daily
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.Treatment of Deep Venous Thrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Reduction in the Risk of Recurrence of Deep VenousThrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily after previous treatment. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients Following Hip Replacement SurgeryFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 110 mg taken orally 1 to 4 hours after surgery and after hemostasis has been achieved, then 220 mg taken once daily for 28 to 35 days. If dabigatran etexilate is not started on the day of surgery, after hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Dosage and Administration (2.4), Use in Specific Populations (8.6) and Clinical Pharmacology (12.2,12.3)].
- For patients with CrCl >30 mL/min: 150 mg orally, twice daily after 5 to 10 days of parenteral anticoagulation (
- Reduction in the Risk of Recurrence of DVT and PE:in Adult Patients
- For patients with CrCl >30 mL/min: 150 mg orally, twice daily after previous treatment ()
2.2 Recommended Dabigatran Etexilate Capsules Dosage for AdultsIndicationDosageReduction in Risk of Stroke and Systemic Embolism in Non-valvular AFCrCl >30 mL/min: 150 mg twice daily CrCl 15 to 30 mL/min: 75 mg twice daily CrCl <15 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl 30 to 50 mL/min with concomitant use of P-gp inhibitors: Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. CrCl <30 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Treatment of DVT and PECrCl >30 mL/min: 150 mg twice daily Reduction in the Risk of Recurrence of DVT and PECrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Prophylaxis of DVT and PE Following Hip Replacement SurgeryCrCl >30 mL/min: 110 mg for first day, then 220 mg once daily CrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors : Avoid co-administration Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation in Adult PatientsFor patients with creatinine clearance (CrCl) >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15 to 30 mL/min), the recommended dosage of dabigatran etexilate capsules is 75 mg twice daily
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.Treatment of Deep Venous Thrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Reduction in the Risk of Recurrence of Deep VenousThrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily after previous treatment. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients Following Hip Replacement SurgeryFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 110 mg taken orally 1 to 4 hours after surgery and after hemostasis has been achieved, then 220 mg taken once daily for 28 to 35 days. If dabigatran etexilate is not started on the day of surgery, after hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Dosage and Administration (2.4), Use in Specific Populations (8.6) and Clinical Pharmacology (12.2,12.3)].
- For patients with CrCl >30 mL/min: 150 mg orally, twice daily after previous treatment (
- Prophylaxis of DVT and PE Following Hip Replacement Surgeryin Adult Patients:
- For patients with CrCl >30 mL/min: 110 mg orally first day, then 220 mg once daily ()
2.2 Recommended Dabigatran Etexilate Capsules Dosage for AdultsIndicationDosageReduction in Risk of Stroke and Systemic Embolism in Non-valvular AFCrCl >30 mL/min: 150 mg twice daily CrCl 15 to 30 mL/min: 75 mg twice daily CrCl <15 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl 30 to 50 mL/min with concomitant use of P-gp inhibitors: Reduce dosage to 75 mg twice daily if given with P-gp inhibitors dronedarone or systemic ketoconazole. CrCl <30 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Treatment of DVT and PECrCl >30 mL/min: 150 mg twice daily Reduction in the Risk of Recurrence of DVT and PECrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors: Avoid co-administration Prophylaxis of DVT and PE Following Hip Replacement SurgeryCrCl >30 mL/min: 110 mg for first day, then 220 mg once daily CrCl ≤30 mL/min or on dialysis: Dosing recommendations cannot be provided CrCl <50 mL/min with concomitant use of P-gp inhibitors : Avoid co-administration Reduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial Fibrillation in Adult PatientsFor patients with creatinine clearance (CrCl) >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily. For patients with severe renal impairment (CrCl 15 to 30 mL/min), the recommended dosage of dabigatran etexilate capsules is 75 mg twice daily
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)]. Dosing recommendations for patients with a CrCl <15 mL/min or on dialysis cannot be provided.Treatment of Deep Venous Thrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily, after 5 to 10 days of parenteral anticoagulation. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Reduction in the Risk of Recurrence of Deep VenousThrombosis and Pulmonary Embolismin Adult PatientsFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 150 mg taken orally, twice daily after previous treatment. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)].Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism in Adult Patients Following Hip Replacement SurgeryFor patients with CrCl >30 mL/min, the recommended dosage of dabigatran etexilate capsules is 110 mg taken orally 1 to 4 hours after surgery and after hemostasis has been achieved, then 220 mg taken once daily for 28 to 35 days. If dabigatran etexilate is not started on the day of surgery, after hemostasis has been achieved initiate treatment with 220 mg once daily. Dosing recommendations for patients with a CrCl ≤30 mL/min or on dialysis cannot be provided
[see Dosage and Administration (2.4), Use in Specific Populations (8.6) and Clinical Pharmacology (12.2,12.3)].
- Treatment of Pediatric VTE:
- For pediatric patients:weight-based dosage, twice daily after at least 5 days of parenteral anticoagulant ()
2.3 Recommended Dabigatran Etexilate Capsules Dosage for PediatricsDabigatran etexilate capsules can be used in pediatric patients aged 8 to less than 18 years of age who are able to swallow the capsules whole. Other age-appropriate pediatric dosage forms of dabigatran etexilate are available for pediatric patients less than 8 years of age. For the treatment of VTE in pediatric patients, initiate treatment following treatment with a parenteral anticoagulant for at least 5 days. For reduction in risk of recurrence of VTE, initiate treatment following previous treatment.
Dabigatran etexilate capsules is dosed orally twice daily, one dose in the morning and one dose in the evening, at approximately the same time every day. The dosing interval should be as close to 12 hours as possible.
The recommended dosage of dabigatran etexilate capsules for the treatment of or reducing the risk of VTE in pediatric patients 8 to less than 18 years of age is based on the patient’s actual weight as shown in Table 1 below. Administer dabigatran etexilate twice daily. Adjust the dosage according to actual weight as treatment progresses [see
Dosage and Administration].Table 1 Weight-BasedDabigatranEtexilate CapsulesDosage for Pediatric Patients Aged 8 to Less Than 18 YearsActual Weight (kg)Dosage (mg)Number of Capsules Needed11 kg to less than 16 kg 75 mg twice daily one 75 mg capsule twice daily 16 kg to less than 26 kg 110 mg twice daily one 110 mg capsule twice daily 26 kg to less than 41 kg 150 mg twice daily one 150 mg capsule twice dailyortwo 75 mg capsules twice daily 41 kg to less than 61 kg 185 mg twice daily one 110 mg capsule plus one 75 mg capsule twice daily 61 kg to less than 81 kg 220 mg twice daily two 110 mg capsule twice daily 81 kg or greater 260 mg twice daily one 150 mg capsule plus one 110 mg capsule twice daily
or
one 110 mg capsule plus two 75 mg capsules twice daily
- Reduction in the Risk of Recurrence of Pediatric VTE:
For pediatric patients: weight-based dosage, twice daily after previous treatment (
)2.3 Recommended Dabigatran Etexilate Capsules Dosage for PediatricsDabigatran etexilate capsules can be used in pediatric patients aged 8 to less than 18 years of age who are able to swallow the capsules whole. Other age-appropriate pediatric dosage forms of dabigatran etexilate are available for pediatric patients less than 8 years of age. For the treatment of VTE in pediatric patients, initiate treatment following treatment with a parenteral anticoagulant for at least 5 days. For reduction in risk of recurrence of VTE, initiate treatment following previous treatment.
Dabigatran etexilate capsules is dosed orally twice daily, one dose in the morning and one dose in the evening, at approximately the same time every day. The dosing interval should be as close to 12 hours as possible.
The recommended dosage of dabigatran etexilate capsules for the treatment of or reducing the risk of VTE in pediatric patients 8 to less than 18 years of age is based on the patient’s actual weight as shown in Table 1 below. Administer dabigatran etexilate twice daily. Adjust the dosage according to actual weight as treatment progresses [see
Dosage and Administration].Table 1 Weight-BasedDabigatranEtexilate CapsulesDosage for Pediatric Patients Aged 8 to Less Than 18 YearsActual Weight (kg)Dosage (mg)Number of Capsules Needed11 kg to less than 16 kg 75 mg twice daily one 75 mg capsule twice daily 16 kg to less than 26 kg 110 mg twice daily one 110 mg capsule twice daily 26 kg to less than 41 kg 150 mg twice daily one 150 mg capsule twice dailyortwo 75 mg capsules twice daily 41 kg to less than 61 kg 185 mg twice daily one 110 mg capsule plus one 75 mg capsule twice daily 61 kg to less than 81 kg 220 mg twice daily two 110 mg capsule twice daily 81 kg or greater 260 mg twice daily one 150 mg capsule plus one 110 mg capsule twice daily
or
one 110 mg capsule plus two 75 mg capsules twice daily
Dabigatran etexilate capsules are NOT substitutable on a milligram-to-milligram basis with other dabigatran etexilate dosage forms.
Review recommendations for converting to or from other oral or parenteral anticoagulants (
,2.6 Converting from or to WarfarinWhen converting patients from warfarin therapy to dabigatran etexilate capsules, discontinue warfarin and start dabigatran etexilate capsules when the INR is below 2.
When converting from dabigatran etexilate capsules to warfarin, adjust the starting time of warfarin as follows:
AdultsFor CrCl ≥50 mL/min, start warfarin 3 days before discontinuing dabigatran etexilate capsules.
For CrCl 30 to 50 mL/min, start warfarin 2 days before discontinuing dabigatran etexilate capsules.
For CrCl 15 to 30 mL/min, start warfarin 1 day before discontinuing dabigatran etexilate capsules.
For CrCl <15 mL/min, no recommendations can be made.
Pediatrics• For eGFR ≥50 mL/min/1.73m2, start warfarin 3 days before discontinuing dabigatran etexilate capsules.
• Pediatric patients with an eGFR <50 mL/min/1.73m2have not been studied. Avoid use of dabigatran etexilate capsules in these patients.
Because dabigatran etexilate capsules can increase INR, the INR will better reflect warfarin’s effect only after dabigatran etexilate capsules have been stopped for at least 2 days
[see Clinical Pharmacology ].)2.7 Converting from or to Parenteral AnticoagulantsFor adult and pediatric patients currently receiving a parenteral anticoagulant, start dabigatran etexilate capsules 0 to 2 hours before the time that the next dose of the parenteral drug was to have been administered or at the time of discontinuation of a continuously administered parenteral drug (e.g., intravenous unfractionated heparin).
For adult patients currently taking dabigatran etexilate capsules wait 12 hours (CrCl ≥30 mL/min) or 24 hours (CrCl <30 mL/min) after the last dose of dabigatran etexilate capsules before initiating treatment with a parenteral anticoagulant
[see Clinical Pharmacology ].For pediatric patients currently taking dabigatran etexilate, wait 12 hours after the last dose before switching to a parenteral anticoagulant.
Temporarily discontinue dabigatran etexilate before invasive or surgical procedures when possible, then restart promptly (
)2.8 Discontinuation for Surgery and Other InterventionsIf possible, discontinue dabigatran etexilate capsules in adults1 to 2 days (CrCl ≥50 mL/min) or 3 to 5 days (CrCl <50 mL/min) before invasive or surgical procedures because of the increased risk of bleeding. Consider longer times for patients undergoing major surgery, spinal puncture, or placement of a spinal or epidural catheter or port, in whom complete hemostasis may be required
[see Use in Specific Populationsand Clinical Pharmacology ].For pediatric patients, discontinue dabigatran etexilate capsules 24 hours before an elective surgery (eGFR > 80 mL/min/1.73m2) or 2 days before an elective surgery (eGFR 50 to 80 mL/min/1.73m2). Pediatric patients with an eGFR <50 mL/min/1.73m2have not been studied, avoid use of dabigatran etexilate capsules in these patients.
If surgery cannot be delayed, there is an increased risk of bleeding [see
Warnings and Precautions]. This risk of bleeding should be weighed against the urgency of intervention [seeWarnings and Precautions]. Use a specific reversal agent (idarucizumab) in case of emergency surgery or urgent procedures when reversal of the anticoagulant effect of dabigatran is needed in adults.Efficacy and safety of idarucizumab have not been established in pediatric patients [seeWarnings and Precautions]. Refer to the idarucizumab prescribing information for additional information. Restart dabigatran etexilate capsules as soon as medically appropriate.
Dabigatran etexilate capsules, 75 mg are white to yellow coloured pellets filled in HPMC capsule shell with white opaque cap and white opaque body imprinted ‘75’ on cap with black ink.
Dabigatran etexilate capsules, 110 mg are white to yellow coloured pellets filled in HPMC capsule shell with white opaque cap and white opaque body imprinted ‘110’ on cap with black ink.
Dabigatran etexilate capsules, 150 mg are white to yellow coloured pellets filled in HPMC capsule shell with white opaque cap and white opaque body imprinted ‘150’ on cap with black ink.
Dabigatran etexilate is contraindicated in patients with:
- Active pathological bleeding [see Warnings and Precautions () and Adverse Reactions (
5.2 Risk of BleedingDabigatran etexilate increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue dabigatran etexilate capsules in patients with active pathological bleeding
[see Dosage and Administration ].Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). Dabigatran etexilate’s anticoagulant activity and half-life are increased in patients with renal impairment
[see Clinical Pharmacology ].Reversal of Anticoagulant Effect:In adults, a specific reversal agent (idarucizumab) for dabigatran etexilate is available when reversal of the anticoagulant effect of dabigatran is needed:
• For emergency surgery/urgent procedures
• In life-threatening or uncontrolled bleeding
In pediatric patients, the efficacy and safety of idarucizumab have not been established.
Hemodialysis can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited
[see Overdosage ]. Prothrombin complex concentrates, or recombinant Factor VIIa may be considered but their use has not been evaluated in clinical trials. Protamine sulfate and vitamin K are not expected to affect the anticoagulant activity of dabigatran. Consider administration of platelet concentrates in cases where thrombocytopenia is present or long-acting antiplatelet drugs have been used.)].6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult TrialsReduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial FibrillationThe RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study provided safety information on the use of two doses of dabigatran etexilate capsules and warfarin
[see Clinical Studies ]. The numbers of patients and their exposures are described in Table 2. Limited information is presented on the 110 mg dosing arm because this dose is not approved.Table 2 Summary of Treatment Exposure in RE-LYDabigatranEtexilate Capsules110 mgtwice dailyDabigatranEtexilate Capsules
150 mgtwice dailyWarfarinTotal number treated 5,983 6,059 5,998 Exposure > 12 months 4,936 4,939 5,193 > 24 months 2,387 2,405 2,470 Mean exposure (months) 20.5 20.3 21.3 Total patient-years 10,242 10,261 10,659 Drug Discontinuation in RE-LYThe rates of adverse reactions leading to treatment discontinuation were 21% for dabigatran etexilate capsules,150 mg and 16% for warfarin. The most frequent adverse reactions leading to discontinuation of dabigatran etexilate capsules were bleeding and gastrointestinal events (i.e., dyspepsia, nausea, upper abdominal pain, gastrointestinal hemorrhage, and diarrhea).
Bleeding[see Warnings and Precautions]Table 3 shows the number of adjudicated major bleeding events during the treatment period in the RE-LY study, with the bleeding rate per 100 subject-years (%). Major bleeding is defined as bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 grams/dL, a transfusion of ≥2 units of packed red blood cells, bleeding at a critical site or with a fatal outcome. Intracranial hemorrhage included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
Table 3 Adjudicated Major Bleeding Events in Treated PatientsaEventDabigatranEtexilateCapsules150 mgN = 6,059n (%/yearb)WarfarinN = 5,998n (%/yearb)DabigatranEtexilate Capsules150 mgvs. WarfarinHR (95% CI)Major Bleedingc 350 (3.47) 374 (3.58) 0.97 (0.84, 1.12) Intracranial Hemorrhage (ICH)d 23 (0.22) 82 (0.77) 0.29 (0.18, 0.46) Hemorrhagic Strokee 6 (0.06) 40 (0.37) 0.16 (0.07, 0.37) Other ICH 17 (0.17) 46 (0.43) 0.38 (0.22, 0.67) Gastrointestinal 162 (1.59) 111 (1.05) 1.51 (1.19, 1.92) Fatal Bleedingf 7 (0.07) 16 (0.15) 0.45 (0.19, 1.10) ICH 3 (0.03) 9 (0.08) 0.35 (0.09, 1.28) Non-intracranialg 4 (0.04) 7 (0.07) 0.59 (0.17, 2.02) aPatients during treatment or within 2 days of stopping study treatment. Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories.
bAnnual event rate per 100 pt-years = 100 * number of subjects with event/subject-years. Subject-years is defined as cumulative number of days from first drug intake to event date, date of last drug intake + 2, death date (whatever occurred first) across all treated subjects divided by 365.25. In case of recurrent events of the same category, the first event was considered.
cDefined as bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 grams/dL, a transfusion of 2 or more units of packed red blood cells, bleeding at a critical site or with fatal outcome.
dIntracranial bleed included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
eOn-treatment analysis based on the safety population, compared to ITT analysis presented in Section 14 Clinical Studies.
fFatal bleed: Adjudicated major bleed as defined above with investigator reported fatal outcome and adjudicated death with primary cause from bleeding.
gNon-intracranial fatal bleed: Adjudicated major bleed as defined above and adjudicated death with primary cause from bleeding but without symptomatic intracranial bleed based on investigator’s clinical assessment.
There was a higher rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules, 150 mg than in patients receiving warfarin (6.6% vs. 4.2%, respectively).
The risk of major bleeds was similar with dabigatran etexilate capsules, 150 mg and warfarin across major subgroups defined by baseline characteristics (see Figure 1), with the exception of age, where there was a trend toward a higher incidence of major bleeding on dabigatran etexilate capsules (hazard ratio 1.2, 95% CI: 1 to 1.5) for patients ≥75 years of age.
Figure 1 Adjudicated Major Bleeding by Baseline Characteristics Including Hemorrhagic Stroke Treated Patients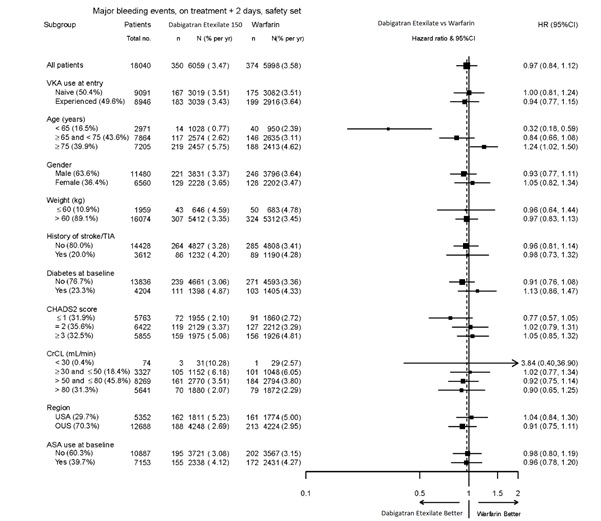
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Gastrointestinal Adverse ReactionsPatients on dabigatran etexilate capsules, 150 mg had an increased incidence of gastrointestinal adverse reactions (35% vs. 24% on warfarin). These were commonly dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) and gastritis-like symptoms (including GERD, esophagitis, erosive gastritis, gastric hemorrhage, hemorrhagic gastritis, hemorrhagic erosive gastritis, and gastrointestinal ulcer).
Hypersensitivity ReactionsIn the RE-LY study, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in <0.1% of patients receiving dabigatran etexilate capsules.
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary EmbolismDabigatran etexilate capsules were studied in 4,387 patients in 4 pivotal, parallel, randomized, double-blind trials. Three of these trials were active-controlled (warfarin) (RE-COVER, RE-COVER II, and RE-MEDY), and one study (RE-SONATE) was placebo-controlled. The demographic characteristics were similar among the 4 pivotal studies and between the treatment groups within these studies. Approximately 60% of the treated patients were male, with a mean age of 55.1 years. The majority of the patients were white (87.7%), 10.3% were Asian, and 1.9% were black with a mean CrCl of 105.6 mL/min.
Bleeding events for the 4 pivotal studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2 grams/dL (1.24 mmol/L or more, or leading to transfusion of 2 or more units of whole blood or red cells).
RE-COVER and RE-COVER II studies compared dabigatran etexilate capsules 150 mg twice daily and warfarin for the treatment of deep vein thrombosis and pulmonary embolism. Patients received 5 to 10 days of an approved parenteral anticoagulant therapy followed by 6 months, with mean exposure of 164 days, of oral only treatment; warfarin was overlapped with parenteral therapy. Table 4 shows the number of patients experiencing bleeding events in the pooled analysis of RE-COVER and RE-COVER II studies during the full treatment including parenteral and oral only treatment periods after randomization.
Table 4 Bleeding Events in RE-COVER and RE-COVER II Treated PatientsBleeding Events-Full Treatment Period Including Parenteral TreatmentDabigatran EtexilateCapsules 150 mgtwice dailyN (%)WarfarinN (%)Hazard Ratio(95% CI)cPatientsN=2,553N=2,554Major bleeding eventa 37 (1.4) 51 (2.0) 0.73 ( 0.48, 1.11) Fatal bleeding 1 (0.04) 2 (0.1) Bleeding in a critical area or organ 7 (0.3) 15 (0.6) Fall in hemoglobin ≥2grams/dL or transfusion
≥2 units of whole
blood or packed red blood cells32 (1.3) 38 (1.5) Bleeding sites for MBEb Intracranial 2 (0.1) 5 (0.2) Retroperitoneal 2 (0.1) 1 (0.04) Intraarticular 2 (0.1) 4 (0.2) Intramuscular 2 (0.1) 6 (0.2) Gastrointestinal 15 (0.6) 14 (0.5) Urogenital 7 (0.3) 14 (0.5) Other 8 (0.3) 8 (0.3) Clinically relevant non-major bleeding 101 (4.0) 170 (6.7) 0.58 (0.46, 0.75) Any bleeding 411 (16.1) 567 (22.7) 0.70 (0.61, 0.79) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
The rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg in the full treatment period was 3.1% (2.4% on warfarin).
The RE-MEDY and RE-SONATE studies provided safety information on the use of dabigatran etexilate capsules for the reduction in the risk of recurrence of deep vein thrombosis and pulmonary embolism.
RE-MEDY was an active-controlled study (warfarin) in which 1,430 patients received dabigatran etexilate capsules 150 mg twice daily following 3 to 12 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-MEDY study had a combined treatment duration of up to more than 3 years, with mean exposure of 473 days. Table 5 shows the number of patients experiencing bleeding events in the study.
Table 5 Bleeding Events in RE-MEDY Treated PatientsDabigatran Etexilate Capsules150 mgtwice dailyN (%)WarfarinN (%)Hazard Ratio(95% CI)cPatientsN=1,430N=1,426Major bleeding eventa 13 (0.9) 25 (1.8) 0.54 (0.25, 1.16) Fatal bleeding 0 1 (0.1) Bleeding in a critical area or organ 7 (0.5) 11 (0.8) Fall in hemoglobin ≥2grams/dL or transfusion ≥2 units
of whole blood or packed red blood cells7 (0.5) 16 (1.1) Bleeding sites for MBEb Intracranial 2 (0.1) 4 (0.3) Intraocular 4 (0.3) 2 (0.1) Retroperitoneal 0 1 (0.1) Intraarticular 0 2 (0.1) Intramuscular 0 4 (0.3) Gastrointestinal 4 (0.3) 8 (0.6) Urogenital 1 (0.1) 1 (0.1) Other 2 (0.1) 4 (0.3) Clinically relevant non-major bleeding 71 (5.0) 125 (8.8) 0.56 (0.42, 0.75) Any bleeding 278 (19.4) 373 (26.2) 0.71 (0.61, 0.83) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
In the RE-MEDY study, the rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg was 3.1% (2.2% on warfarin).
RE-SONATE was a placebo-controlled study in which 684 patients received dabigatran etexilate capsules, 150 mg twice daily following 6 to 18 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-SONATE study had combined treatment duration up to 9 months, with mean exposure of 165 days. Table 6 shows the number of patients experiencing bleeding events in the study.
Table 6 Bleeding Events in RE-SONATE Treated PatientsDabigatran
Etexilate Capsules150 mgtwice dailyN (%)PlaceboN (%)Hazard Ratio(95% CI)cPtientsN=684N=659Major bleeding eventa 2 (0.3) 0 Bleeding in a critical area or organ 0 0 Gastrointestinalb 2 (0.3) 0 Clinically relevant non-major bleeding 34 (5.0) 13 (2.0) 2.54 (1.34, 4.82) Any bleeding 72 (10.5) 40 (6.1) 1.77 (1.20, 2.61) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
In the RE-SONATE study, the rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg was 0.7% (0.3% on placebo).
Clinical Myocardial Infarction EventsIn the active-controlled VTE studies, a higher rate of clinical myocardial infarction was reported in patients who received dabigatran etexilate capsules [20 (0.66 per 100 patient-years)] than in those who received warfarin [5 (0.17 per 100 patient-years)]. In the placebo-controlled study, a similar rate of nonfatal and fatal clinical myocardial infarction was reported in patients who received dabigatran etexilate capsules [1 (0.32 per 100 patient-years)] and in those who received placebo [1 (0.34 per 100 patient-years)].
Gastrointestinal Adverse ReactionsIn the four pivotal studies, patients on dabigatran etexilate capsules 150 mg had a similar incidence of gastrointestinal adverse reactions (24.7% vs. 22.7% on warfarin). Dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) occurred in patients on dabigatran etexilate capsules 7.5% vs. 5.5% on warfarin, and gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive gastritis and gastric hemorrhage) occurred at 3% vs. 1.7%, respectively.
Hypersensitivity ReactionsIn the 4 pivotal studies, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in 0.1% of patients receiving dabigatran etexilate capsules.
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism Following Hip Replacement SurgeryDabigatran etexilate capsules were studied in 5,476 patients, randomized and treated in two double-blind, active-controlled non-inferiority trials (RE-NOVATE and RE-NOVATE II). The demographic characteristics were similar across the two studies and between the treatment groups within these studies. Approximately 45.3% of the treated patients were male, with a mean age of 63.2 years. The majority of the patients were white (96.1%), 3.6% were Asian, and 0.3% were black with a mean CrCl of 92 mL/min.
Bleeding events for the RE-NOVATE and RE-NOVATE II studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or retroperitoneal bleeding), bleeding causing a fall in hemoglobin level of 2 grams/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells, requiring treatment cessation or leading to re-operation.
The RE-NOVATE study compared dabigatran etexilate capsules 75 mg taken orally 1 to 4 hours after surgery followed by 150 mg once daily, dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. The RE-NOVATE II study compared dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. In the RE-NOVATE and RE-NOVATE II studies, patients received 28 to 35 days of dabigatran etexilate capsules or enoxaparin with median exposure of 33 days. Tables 7 and 8 show the number of patients experiencing bleeding events in the analysis of RE-NOVATE and RE-NOVATE II.
Table 7 Bleeding Events in RE-NOVATE Treated PatientsDabigatran Etexilate Capsules 220 mgN (%)EnoxaparinN (%)PatientsN=1,146N=1,154Major bleeding event 23 (2.0) 18 (1.6) Clinically relevant non-major bleeding 48 (4.2) 40 (3.5) Any bleeding 141 (12.3) 132 (11.4) Table 8 Bleeding Events in RE-NOVATE II Treated PatientsDabigatran Etexilate Capsules 220 mgN (%)EnoxaparinN (%)PatientsN=1,010N=1,003Major bleeding event 14 (1.4) 9 (0.9) Clinically relevant non-major bleeding 26 (2.6) 20 (2.0) Any bleeding 98 (9.7) 83 (8.3) In the two studies, the rate of major gastrointestinal bleeds in patients receiving dabigatran etexilate capsules and enoxaparin was the same (0.1%) and for any gastrointestinal bleeds was 1.4% for dabigatran etexilate capsules 220 mg and 0.9% for enoxaparin.
Gastrointestinal Adverse ReactionsIn the two studies, the incidence of gastrointestinal adverse reactions for patients on dabigatran etexilate capsules 220 mg and enoxaparin was 39.5% and 39.5%, respectively. Dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) occurred in patients on dabigatran etexilate capsules 220 mg in 4.1% vs. 3.8% on enoxaparin, and gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive gastritis and gastric hemorrhage) occurred at 0.6% vs. 1%, respectively.
HypersensitivityReactionsIn the two studies, drug hypersensitivity (such as urticaria, rash, and pruritus) was reported in 0.3% of patients receiving dabigatran etexilate capsules 220 mg.
Clinical Myocardial Infarction EventsIn the two studies, clinical myocardial infarction was reported in 2 (0.1%) of patients who received dabigatran etexilate capsules 220 mg and 6 (0.3%) of patients who received enoxaparin.
Pediatric Trials
Treatment of VTE in Pediatric PatientsThe safety of dabigatran etexilate in the treatment of VTE in pediatric patients was studied in one phase III trial (DIVERSITY). The DIVERSITY study was a randomized, open-label, active-controlled, parallel-group trial comparing dabigatran etexilate with standard of care – SOC (vitamin K antagonists, low molecular weight heparin, or fondaparinux). There were 266 pediatric patients who received study treatment, 176 patients treated with dabigatran etexilate and 90 patients treated with SOC. Patients on dabigatran etexilate received age- and weight-adjusted dosages of an age-appropriate formulation of dabigatran etexilate (capsules, pellets, or oral solution) twice daily.
Patients had a median age of 14 years (range: 0 to 17 years), 92% were white, and half the patients were male (50%). Following at least 5 days of parenteral anticoagulant therapy, the median duration of treatment with dabigatran etexilate was 85 days (range: 1 to 105). Patients with estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73m2were excluded from the trial.
BleedingData on adjudicated major bleeding, clinically relevant non-major (CRNM) bleeding and minor bleeding events, for the dabigatran etexilate group and the SOC group in the DIVERSITY study, are reported in Table 9. There was no statistically significant difference in the time to first major bleeding event.
Table 9 Summary of All Adjudicated Bleeding Events During On-Treatment Period in DIVERSITYDabigatranEtexilateN (%)Standard of Care (SOC)N (%)PatientsN=176N=90Major bleeding event1 4 (2.3) 2 (2.2) Fatal bleeding 0 1 (1.1) Clinically relevant non-major bleeding 2 (1.1) 1 (1.1) Minor bleeding 33 (19) 21 (23) Major and clinically relevant non-major bleeding 6 (3.4) 3 (3.3) Any bleeding 38 (22) 22 (24) 1Major bleeding event if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells.
Site-specific bleeding rates were comparable between the two arms, with the exception of the rate of any gastrointestinal bleeds (5.7% in dabigatran etexilate arm vs. 1.8% in SOC arm).
Gastrointestinal Adverse ReactionsThe incidence of gastrointestinal adverse reactions for patients on dabigatran etexilate and SOC was 32% and 12%, respectively, with the following occurring in ≥5% of patients taking dabigatran etexilate: dyspepsia (including term gastro-esophageal reflux disease, gastric pH decreased and esophagitis) in 9% (vs. 2%), upper abdominal pain in 5% (vs. 1%), vomiting in 8% (vs. 2%), nausea 5% (vs. 4%), and diarrhea 5% (vs. 1%).
Reduction in Risk of Recurrence of VTE in Pediatric PatientsThe safety of dabigatran etexilate in the reduction in the risk of recurrence of VTE in pediatric patients was studied in one open-label single-arm trial (Study 2). Study 2 enrolled patients who required further anticoagulation due to the presence of a clinical risk factor after completing the initial treatment for confirmed VTE (for at least 3 months) or after completing the DIVERSITY study and received dabigatran etexilate until the clinical risk factor resolved, or up to a maximum of 12 months. There were 213 pediatric patients treated with dabigatran etexilate, in a similar fashion as in the DIVERSITY trial.
Patients previously enrolled on DIVERSITY accounted for 43% of patients enrolled on Study 2 (29% from dabigatran etexilate arm and 14% from SOC arm). The median duration of treatment with dabigatran etexilate in Study 2 was 42 weeks (range: 0 to 56 weeks), with 45% of patients completing the 12 months planned duration, 17% stopping due to resolution of VTE risk factors, 12% stopping due to failure to attain target dabigatran concentration and 6% had an adverse event leading to discontinuation.
During the on-treatment period of Study 2, 3 patients (1.4%) had a major bleeding event, 3 patients (1.4%) had a clinically relevant non-major bleeding event, and 44 patients (20%) had a minor bleeding event. The most common drug-related adverse reactions were dyspepsia (5%), epistaxis (3.3%), nausea (3.3%) and menorrhagia (2.8%).
The adverse reaction profile in pediatric patients was generally consistent with that of adult patients.
- History of a serious hypersensitivity reaction to dabigatran, dabigatran etexilate, or to one of the excipients of the product (e.g., anaphylactic reaction or anaphylactic shock) [see Adverse Reactions ()].
6.1 Clinical Trials ExperienceBecause clinical trials are conducted under widely varying conditions, adverse reactions rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult TrialsReduction of Risk of Stroke and Systemic Embolism in Non-valvular Atrial FibrillationThe RE-LY (Randomized Evaluation of Long-term Anticoagulant Therapy) study provided safety information on the use of two doses of dabigatran etexilate capsules and warfarin
[see Clinical Studies ]. The numbers of patients and their exposures are described in Table 2. Limited information is presented on the 110 mg dosing arm because this dose is not approved.Table 2 Summary of Treatment Exposure in RE-LYDabigatranEtexilate Capsules110 mgtwice dailyDabigatranEtexilate Capsules
150 mgtwice dailyWarfarinTotal number treated 5,983 6,059 5,998 Exposure > 12 months 4,936 4,939 5,193 > 24 months 2,387 2,405 2,470 Mean exposure (months) 20.5 20.3 21.3 Total patient-years 10,242 10,261 10,659 Drug Discontinuation in RE-LYThe rates of adverse reactions leading to treatment discontinuation were 21% for dabigatran etexilate capsules,150 mg and 16% for warfarin. The most frequent adverse reactions leading to discontinuation of dabigatran etexilate capsules were bleeding and gastrointestinal events (i.e., dyspepsia, nausea, upper abdominal pain, gastrointestinal hemorrhage, and diarrhea).
Bleeding[see Warnings and Precautions]Table 3 shows the number of adjudicated major bleeding events during the treatment period in the RE-LY study, with the bleeding rate per 100 subject-years (%). Major bleeding is defined as bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 grams/dL, a transfusion of ≥2 units of packed red blood cells, bleeding at a critical site or with a fatal outcome. Intracranial hemorrhage included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
Table 3 Adjudicated Major Bleeding Events in Treated PatientsaEventDabigatranEtexilateCapsules150 mgN = 6,059n (%/yearb)WarfarinN = 5,998n (%/yearb)DabigatranEtexilate Capsules150 mgvs. WarfarinHR (95% CI)Major Bleedingc 350 (3.47) 374 (3.58) 0.97 (0.84, 1.12) Intracranial Hemorrhage (ICH)d 23 (0.22) 82 (0.77) 0.29 (0.18, 0.46) Hemorrhagic Strokee 6 (0.06) 40 (0.37) 0.16 (0.07, 0.37) Other ICH 17 (0.17) 46 (0.43) 0.38 (0.22, 0.67) Gastrointestinal 162 (1.59) 111 (1.05) 1.51 (1.19, 1.92) Fatal Bleedingf 7 (0.07) 16 (0.15) 0.45 (0.19, 1.10) ICH 3 (0.03) 9 (0.08) 0.35 (0.09, 1.28) Non-intracranialg 4 (0.04) 7 (0.07) 0.59 (0.17, 2.02) aPatients during treatment or within 2 days of stopping study treatment. Major bleeding events within each subcategory were counted once per patient, but patients may have contributed events to multiple subcategories.
bAnnual event rate per 100 pt-years = 100 * number of subjects with event/subject-years. Subject-years is defined as cumulative number of days from first drug intake to event date, date of last drug intake + 2, death date (whatever occurred first) across all treated subjects divided by 365.25. In case of recurrent events of the same category, the first event was considered.
cDefined as bleeding accompanied by one or more of the following: a decrease in hemoglobin of ≥2 grams/dL, a transfusion of 2 or more units of packed red blood cells, bleeding at a critical site or with fatal outcome.
dIntracranial bleed included intracerebral (hemorrhagic stroke), subarachnoid, and subdural bleeds.
eOn-treatment analysis based on the safety population, compared to ITT analysis presented in Section 14 Clinical Studies.
fFatal bleed: Adjudicated major bleed as defined above with investigator reported fatal outcome and adjudicated death with primary cause from bleeding.
gNon-intracranial fatal bleed: Adjudicated major bleed as defined above and adjudicated death with primary cause from bleeding but without symptomatic intracranial bleed based on investigator’s clinical assessment.
There was a higher rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules, 150 mg than in patients receiving warfarin (6.6% vs. 4.2%, respectively).
The risk of major bleeds was similar with dabigatran etexilate capsules, 150 mg and warfarin across major subgroups defined by baseline characteristics (see Figure 1), with the exception of age, where there was a trend toward a higher incidence of major bleeding on dabigatran etexilate capsules (hazard ratio 1.2, 95% CI: 1 to 1.5) for patients ≥75 years of age.
Figure 1 Adjudicated Major Bleeding by Baseline Characteristics Including Hemorrhagic Stroke Treated Patients
Note: The figure above presents effects in various subgroups all of which are baseline characteristics and all of which were pre-specified. The 95% confidence limits that are shown do not take into account how many comparisons were made, nor do they reflect the effect of a particular factor after adjustment for all other factors. Apparent homogeneity or heterogeneity among groups should not be over-interpreted.
Gastrointestinal Adverse ReactionsPatients on dabigatran etexilate capsules, 150 mg had an increased incidence of gastrointestinal adverse reactions (35% vs. 24% on warfarin). These were commonly dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) and gastritis-like symptoms (including GERD, esophagitis, erosive gastritis, gastric hemorrhage, hemorrhagic gastritis, hemorrhagic erosive gastritis, and gastrointestinal ulcer).
Hypersensitivity ReactionsIn the RE-LY study, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in <0.1% of patients receiving dabigatran etexilate capsules.
Treatment and Reduction in the Risk of Recurrence of Deep Venous Thrombosis and Pulmonary EmbolismDabigatran etexilate capsules were studied in 4,387 patients in 4 pivotal, parallel, randomized, double-blind trials. Three of these trials were active-controlled (warfarin) (RE-COVER, RE-COVER II, and RE-MEDY), and one study (RE-SONATE) was placebo-controlled. The demographic characteristics were similar among the 4 pivotal studies and between the treatment groups within these studies. Approximately 60% of the treated patients were male, with a mean age of 55.1 years. The majority of the patients were white (87.7%), 10.3% were Asian, and 1.9% were black with a mean CrCl of 105.6 mL/min.
Bleeding events for the 4 pivotal studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2 grams/dL (1.24 mmol/L or more, or leading to transfusion of 2 or more units of whole blood or red cells).
RE-COVER and RE-COVER II studies compared dabigatran etexilate capsules 150 mg twice daily and warfarin for the treatment of deep vein thrombosis and pulmonary embolism. Patients received 5 to 10 days of an approved parenteral anticoagulant therapy followed by 6 months, with mean exposure of 164 days, of oral only treatment; warfarin was overlapped with parenteral therapy. Table 4 shows the number of patients experiencing bleeding events in the pooled analysis of RE-COVER and RE-COVER II studies during the full treatment including parenteral and oral only treatment periods after randomization.
Table 4 Bleeding Events in RE-COVER and RE-COVER II Treated PatientsBleeding Events-Full Treatment Period Including Parenteral TreatmentDabigatran EtexilateCapsules 150 mgtwice dailyN (%)WarfarinN (%)Hazard Ratio(95% CI)cPatientsN=2,553N=2,554Major bleeding eventa 37 (1.4) 51 (2.0) 0.73 ( 0.48, 1.11) Fatal bleeding 1 (0.04) 2 (0.1) Bleeding in a critical area or organ 7 (0.3) 15 (0.6) Fall in hemoglobin ≥2grams/dL or transfusion
≥2 units of whole
blood or packed red blood cells32 (1.3) 38 (1.5) Bleeding sites for MBEb Intracranial 2 (0.1) 5 (0.2) Retroperitoneal 2 (0.1) 1 (0.04) Intraarticular 2 (0.1) 4 (0.2) Intramuscular 2 (0.1) 6 (0.2) Gastrointestinal 15 (0.6) 14 (0.5) Urogenital 7 (0.3) 14 (0.5) Other 8 (0.3) 8 (0.3) Clinically relevant non-major bleeding 101 (4.0) 170 (6.7) 0.58 (0.46, 0.75) Any bleeding 411 (16.1) 567 (22.7) 0.70 (0.61, 0.79) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
The rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg in the full treatment period was 3.1% (2.4% on warfarin).
The RE-MEDY and RE-SONATE studies provided safety information on the use of dabigatran etexilate capsules for the reduction in the risk of recurrence of deep vein thrombosis and pulmonary embolism.
RE-MEDY was an active-controlled study (warfarin) in which 1,430 patients received dabigatran etexilate capsules 150 mg twice daily following 3 to 12 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-MEDY study had a combined treatment duration of up to more than 3 years, with mean exposure of 473 days. Table 5 shows the number of patients experiencing bleeding events in the study.
Table 5 Bleeding Events in RE-MEDY Treated PatientsDabigatran Etexilate Capsules150 mgtwice dailyN (%)WarfarinN (%)Hazard Ratio(95% CI)cPatientsN=1,430N=1,426Major bleeding eventa 13 (0.9) 25 (1.8) 0.54 (0.25, 1.16) Fatal bleeding 0 1 (0.1) Bleeding in a critical area or organ 7 (0.5) 11 (0.8) Fall in hemoglobin ≥2grams/dL or transfusion ≥2 units
of whole blood or packed red blood cells7 (0.5) 16 (1.1) Bleeding sites for MBEb Intracranial 2 (0.1) 4 (0.3) Intraocular 4 (0.3) 2 (0.1) Retroperitoneal 0 1 (0.1) Intraarticular 0 2 (0.1) Intramuscular 0 4 (0.3) Gastrointestinal 4 (0.3) 8 (0.6) Urogenital 1 (0.1) 1 (0.1) Other 2 (0.1) 4 (0.3) Clinically relevant non-major bleeding 71 (5.0) 125 (8.8) 0.56 (0.42, 0.75) Any bleeding 278 (19.4) 373 (26.2) 0.71 (0.61, 0.83) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
In the RE-MEDY study, the rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg was 3.1% (2.2% on warfarin).
RE-SONATE was a placebo-controlled study in which 684 patients received dabigatran etexilate capsules, 150 mg twice daily following 6 to 18 months of oral anticoagulant regimen. Patients in the treatment studies who rolled over into the RE-SONATE study had combined treatment duration up to 9 months, with mean exposure of 165 days. Table 6 shows the number of patients experiencing bleeding events in the study.
Table 6 Bleeding Events in RE-SONATE Treated PatientsDabigatran
Etexilate Capsules150 mgtwice dailyN (%)PlaceboN (%)Hazard Ratio(95% CI)cPtientsN=684N=659Major bleeding eventa 2 (0.3) 0 Bleeding in a critical area or organ 0 0 Gastrointestinalb 2 (0.3) 0 Clinically relevant non-major bleeding 34 (5.0) 13 (2.0) 2.54 (1.34, 4.82) Any bleeding 72 (10.5) 40 (6.1) 1.77 (1.20, 2.61) Note: MBE can belong to more than one criterion.
aPatients with at least one MBE.
bBleeding site based on investigator assessment. Patients can have more than one site of bleeding.
cConfidence interval
In the RE-SONATE study, the rate of any gastrointestinal bleeds in patients receiving dabigatran etexilate capsules 150 mg was 0.7% (0.3% on placebo).
Clinical Myocardial Infarction EventsIn the active-controlled VTE studies, a higher rate of clinical myocardial infarction was reported in patients who received dabigatran etexilate capsules [20 (0.66 per 100 patient-years)] than in those who received warfarin [5 (0.17 per 100 patient-years)]. In the placebo-controlled study, a similar rate of nonfatal and fatal clinical myocardial infarction was reported in patients who received dabigatran etexilate capsules [1 (0.32 per 100 patient-years)] and in those who received placebo [1 (0.34 per 100 patient-years)].
Gastrointestinal Adverse ReactionsIn the four pivotal studies, patients on dabigatran etexilate capsules 150 mg had a similar incidence of gastrointestinal adverse reactions (24.7% vs. 22.7% on warfarin). Dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) occurred in patients on dabigatran etexilate capsules 7.5% vs. 5.5% on warfarin, and gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive gastritis and gastric hemorrhage) occurred at 3% vs. 1.7%, respectively.
Hypersensitivity ReactionsIn the 4 pivotal studies, drug hypersensitivity (including urticaria, rash, and pruritus), allergic edema, anaphylactic reaction, and anaphylactic shock were reported in 0.1% of patients receiving dabigatran etexilate capsules.
Prophylaxis of Deep Vein Thrombosis and Pulmonary Embolism Following Hip Replacement SurgeryDabigatran etexilate capsules were studied in 5,476 patients, randomized and treated in two double-blind, active-controlled non-inferiority trials (RE-NOVATE and RE-NOVATE II). The demographic characteristics were similar across the two studies and between the treatment groups within these studies. Approximately 45.3% of the treated patients were male, with a mean age of 63.2 years. The majority of the patients were white (96.1%), 3.6% were Asian, and 0.3% were black with a mean CrCl of 92 mL/min.
Bleeding events for the RE-NOVATE and RE-NOVATE II studies were classified as major bleeding events if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or retroperitoneal bleeding), bleeding causing a fall in hemoglobin level of 2 grams/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells, requiring treatment cessation or leading to re-operation.
The RE-NOVATE study compared dabigatran etexilate capsules 75 mg taken orally 1 to 4 hours after surgery followed by 150 mg once daily, dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. The RE-NOVATE II study compared dabigatran etexilate capsules 110 mg taken orally 1 to 4 hours after surgery followed by 220 mg once daily and subcutaneous enoxaparin 40 mg once daily initiated the evening before surgery for the prophylaxis of deep vein thrombosis and pulmonary embolism in patients who had undergone hip replacement surgery. In the RE-NOVATE and RE-NOVATE II studies, patients received 28 to 35 days of dabigatran etexilate capsules or enoxaparin with median exposure of 33 days. Tables 7 and 8 show the number of patients experiencing bleeding events in the analysis of RE-NOVATE and RE-NOVATE II.
Table 7 Bleeding Events in RE-NOVATE Treated PatientsDabigatran Etexilate Capsules 220 mgN (%)EnoxaparinN (%)PatientsN=1,146N=1,154Major bleeding event 23 (2.0) 18 (1.6) Clinically relevant non-major bleeding 48 (4.2) 40 (3.5) Any bleeding 141 (12.3) 132 (11.4) Table 8 Bleeding Events in RE-NOVATE II Treated PatientsDabigatran Etexilate Capsules 220 mgN (%)EnoxaparinN (%)PatientsN=1,010N=1,003Major bleeding event 14 (1.4) 9 (0.9) Clinically relevant non-major bleeding 26 (2.6) 20 (2.0) Any bleeding 98 (9.7) 83 (8.3) In the two studies, the rate of major gastrointestinal bleeds in patients receiving dabigatran etexilate capsules and enoxaparin was the same (0.1%) and for any gastrointestinal bleeds was 1.4% for dabigatran etexilate capsules 220 mg and 0.9% for enoxaparin.
Gastrointestinal Adverse ReactionsIn the two studies, the incidence of gastrointestinal adverse reactions for patients on dabigatran etexilate capsules 220 mg and enoxaparin was 39.5% and 39.5%, respectively. Dyspepsia (including abdominal pain upper, abdominal pain, abdominal discomfort, and epigastric discomfort) occurred in patients on dabigatran etexilate capsules 220 mg in 4.1% vs. 3.8% on enoxaparin, and gastritis-like symptoms (including gastritis, GERD, esophagitis, erosive gastritis and gastric hemorrhage) occurred at 0.6% vs. 1%, respectively.
HypersensitivityReactionsIn the two studies, drug hypersensitivity (such as urticaria, rash, and pruritus) was reported in 0.3% of patients receiving dabigatran etexilate capsules 220 mg.
Clinical Myocardial Infarction EventsIn the two studies, clinical myocardial infarction was reported in 2 (0.1%) of patients who received dabigatran etexilate capsules 220 mg and 6 (0.3%) of patients who received enoxaparin.
Pediatric Trials
Treatment of VTE in Pediatric PatientsThe safety of dabigatran etexilate in the treatment of VTE in pediatric patients was studied in one phase III trial (DIVERSITY). The DIVERSITY study was a randomized, open-label, active-controlled, parallel-group trial comparing dabigatran etexilate with standard of care – SOC (vitamin K antagonists, low molecular weight heparin, or fondaparinux). There were 266 pediatric patients who received study treatment, 176 patients treated with dabigatran etexilate and 90 patients treated with SOC. Patients on dabigatran etexilate received age- and weight-adjusted dosages of an age-appropriate formulation of dabigatran etexilate (capsules, pellets, or oral solution) twice daily.
Patients had a median age of 14 years (range: 0 to 17 years), 92% were white, and half the patients were male (50%). Following at least 5 days of parenteral anticoagulant therapy, the median duration of treatment with dabigatran etexilate was 85 days (range: 1 to 105). Patients with estimated glomerular filtration rate (eGFR) < 50 mL/min/1.73m2were excluded from the trial.
BleedingData on adjudicated major bleeding, clinically relevant non-major (CRNM) bleeding and minor bleeding events, for the dabigatran etexilate group and the SOC group in the DIVERSITY study, are reported in Table 9. There was no statistically significant difference in the time to first major bleeding event.
Table 9 Summary of All Adjudicated Bleeding Events During On-Treatment Period in DIVERSITYDabigatranEtexilateN (%)Standard of Care (SOC)N (%)PatientsN=176N=90Major bleeding event1 4 (2.3) 2 (2.2) Fatal bleeding 0 1 (1.1) Clinically relevant non-major bleeding 2 (1.1) 1 (1.1) Minor bleeding 33 (19) 21 (23) Major and clinically relevant non-major bleeding 6 (3.4) 3 (3.3) Any bleeding 38 (22) 22 (24) 1Major bleeding event if at least one of the following criteria applied: fatal bleeding, symptomatic bleeding in a critical area or organ (intraocular, intracranial, intraspinal or intramuscular with compartment syndrome, retroperitoneal bleeding, intra-articular bleeding, or pericardial bleeding), bleeding causing a fall in hemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of 2 or more units of whole blood or red cells.
Site-specific bleeding rates were comparable between the two arms, with the exception of the rate of any gastrointestinal bleeds (5.7% in dabigatran etexilate arm vs. 1.8% in SOC arm).
Gastrointestinal Adverse ReactionsThe incidence of gastrointestinal adverse reactions for patients on dabigatran etexilate and SOC was 32% and 12%, respectively, with the following occurring in ≥5% of patients taking dabigatran etexilate: dyspepsia (including term gastro-esophageal reflux disease, gastric pH decreased and esophagitis) in 9% (vs. 2%), upper abdominal pain in 5% (vs. 1%), vomiting in 8% (vs. 2%), nausea 5% (vs. 4%), and diarrhea 5% (vs. 1%).
Reduction in Risk of Recurrence of VTE in Pediatric PatientsThe safety of dabigatran etexilate in the reduction in the risk of recurrence of VTE in pediatric patients was studied in one open-label single-arm trial (Study 2). Study 2 enrolled patients who required further anticoagulation due to the presence of a clinical risk factor after completing the initial treatment for confirmed VTE (for at least 3 months) or after completing the DIVERSITY study and received dabigatran etexilate until the clinical risk factor resolved, or up to a maximum of 12 months. There were 213 pediatric patients treated with dabigatran etexilate, in a similar fashion as in the DIVERSITY trial.
Patients previously enrolled on DIVERSITY accounted for 43% of patients enrolled on Study 2 (29% from dabigatran etexilate arm and 14% from SOC arm). The median duration of treatment with dabigatran etexilate in Study 2 was 42 weeks (range: 0 to 56 weeks), with 45% of patients completing the 12 months planned duration, 17% stopping due to resolution of VTE risk factors, 12% stopping due to failure to attain target dabigatran concentration and 6% had an adverse event leading to discontinuation.
During the on-treatment period of Study 2, 3 patients (1.4%) had a major bleeding event, 3 patients (1.4%) had a clinically relevant non-major bleeding event, and 44 patients (20%) had a minor bleeding event. The most common drug-related adverse reactions were dyspepsia (5%), epistaxis (3.3%), nausea (3.3%) and menorrhagia (2.8%).
The adverse reaction profile in pediatric patients was generally consistent with that of adult patients.
- Mechanical prosthetic heart valve [see Warnings and Precautions (.)]
5.4 Thromboembolic and Bleeding Events in Patients with Prosthetic Heart ValvesThe safety and efficacy of dabigatran etexilate capsules in adult patients with bileaflet mechanical prosthetic heart valves was evaluated in the RE-ALIGN trial, in which patients with bileaflet mechanical prosthetic heart valves (recently implanted or implanted more than three months prior to enrollment) were randomized to dose-adjusted warfarin or 150 mg, 220 mg, or 300 mg of dabigatran etexilate capsules twice a day. RE-ALIGN was terminated early due to the occurrence of significantly more thromboembolic events (valve thrombosis, stroke, transient ischemic attack, and myocardial infarction) and an excess of major bleeding (predominantly post-operative pericardial effusions requiring intervention for hemodynamic compromise) in the dabigatran etexilate capsules treatment arm as compared to the warfarin treatment arm. These bleeding and thromboembolic events were seen both in patients who were initiated on dabigatran etexilate capsules postoperatively within three days of mechanical bileaflet valve implantation, as well as in patients whose valves had been implanted more than three months prior to enrollment. Therefore, the use of dabigatran etexilate is contraindicated in all patients with mechanical prosthetic valves
[see Contraindications ].The use of dabigatran etexilate for the prophylaxis of thromboembolic events in patients with atrial fibrillation in the setting of other forms of valvular heart disease, including the presence of a bioprosthetic heart valve, has not been studied and is not recommended.
The following clinically significant adverse reactions are described elsewhere in the labeling:
• Increased Risk of Thrombotic Events after Premature Discontinuation
Premature discontinuation of any oral anticoagulant, including dabigatran etexilate, in the absence of adequate alternative anticoagulation increases the risk of thrombotic events. If dabigatran etexilate capsules are discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant and restart dabigatran etexilate capsules as soon as medically appropriate
• Risk of Bleeding
Dabigatran etexilate increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue dabigatran etexilate capsules in patients with active pathological bleeding
Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). Dabigatran etexilate’s anticoagulant activity and half-life are increased in patients with renal impairment
In adults, a specific reversal agent (idarucizumab) for dabigatran etexilate is available when reversal of the anticoagulant effect of dabigatran is needed:
• For emergency surgery/urgent procedures
• In life-threatening or uncontrolled bleeding
In pediatric patients, the efficacy and safety of idarucizumab have not been established.
Hemodialysis can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited
• Spinal/Epidural Anesthesia or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal puncture is employed, patients treated with anticoagulant agents are at risk of developing an epidural or spinal hematoma which can result in long-term or permanent paralysis
To reduce the potential risk of bleeding associated with the concurrent use of dabigatran etexilate and epidural or spinal anesthesia/analgesia or spinal puncture, consider the pharmacokinetic profile of dabigatran
Should the physician decide to administer anticoagulation in the context of epidural or spinal anesthesia/analgesia or lumbar puncture, monitor frequently to detect any signs or symptoms of neurological impairment, such as midline back pain, sensory and motor deficits (numbness, tingling, or weakness in lower limbs), bowel and/or bladder dysfunction. Instruct patients to immediately report if they experience any of the above signs or symptoms. If signs or symptoms of spinal hematoma are suspected, initiate urgent diagnosis and treatment including consideration for spinal cord decompression even though such treatment may not prevent or reverse neurological sequelae.
• Thromboembolic and Bleeding Events in Patients with Prosthetic Heart Valves
The safety and efficacy of dabigatran etexilate capsules in adult patients with bileaflet mechanical prosthetic heart valves was evaluated in the RE-ALIGN trial, in which patients with bileaflet mechanical prosthetic heart valves (recently implanted or implanted more than three months prior to enrollment) were randomized to dose-adjusted warfarin or 150 mg, 220 mg, or 300 mg of dabigatran etexilate capsules twice a day. RE-ALIGN was terminated early due to the occurrence of significantly more thromboembolic events (valve thrombosis, stroke, transient ischemic attack, and myocardial infarction) and an excess of major bleeding (predominantly post-operative pericardial effusions requiring intervention for hemodynamic compromise) in the dabigatran etexilate capsules treatment arm as compared to the warfarin treatment arm. These bleeding and thromboembolic events were seen both in patients who were initiated on dabigatran etexilate capsules postoperatively within three days of mechanical bileaflet valve implantation, as well as in patients whose valves had been implanted more than three months prior to enrollment. Therefore, the use of dabigatran etexilate is contraindicated in all patients with mechanical prosthetic valves
The use of dabigatran etexilate for the prophylaxis of thromboembolic events in patients with atrial fibrillation in the setting of other forms of valvular heart disease, including the presence of a bioprosthetic heart valve, has not been studied and is not recommended.
•Increased Risk of Thrombosis in Patients with Triple-Positive Antiphospholipid Syndrome [
Direct-acting oral anticoagulants (DOACs), including dabigatran etexilate, are not recommended for use in patients with triple-positive antiphospholipid syndrome (APS). For patients with APS (especially those who are triple-positive [positive for lupus anticoagulant, anticardiolipin, and anti-beta 2-glycoprotein I antibodies]), treatment with DOACs has been associated with increased rates of recurrent thrombotic events compared with vitamin K antagonist therapy.
The most serious adverse reactions reported with dabigatran etexilate were related to bleeding
Dabigatran etexilate increases the risk of bleeding and can cause significant and, sometimes, fatal bleeding. Promptly evaluate any signs or symptoms of blood loss (e.g., a drop in hemoglobin and/or hematocrit or hypotension). Discontinue dabigatran etexilate capsules in patients with active pathological bleeding
Risk factors for bleeding include the concomitant use of other drugs that increase the risk of bleeding (e.g., anti-platelet agents, heparin, fibrinolytic therapy, and chronic use of NSAIDs). Dabigatran etexilate’s anticoagulant activity and half-life are increased in patients with renal impairment
In adults, a specific reversal agent (idarucizumab) for dabigatran etexilate is available when reversal of the anticoagulant effect of dabigatran is needed:
• For emergency surgery/urgent procedures
• In life-threatening or uncontrolled bleeding
In pediatric patients, the efficacy and safety of idarucizumab have not been established.
Hemodialysis can remove dabigatran; however the clinical experience supporting the use of hemodialysis as a treatment for bleeding is limited
The chemical name for dabigatran etexilate mesylate, a direct thrombin inhibitor, is β-Alanine, Ethyl 3-[[[2-[[[4-[[[(hexyloxy)carbonyl]amino]iminomethyl]phenyl]amino]methyl]-1-methyl-1H benzimidazol-5-yl]carbonyl](pyridine-2-yl)amino]propanoate. The molecular formula is C34H41N7O5·CH4O3S and the molecular weight is 723.86 (mesylate salt), 627.75 (free base). The structural formula is:
Dabigatran etexilate mesylate is a white to yellow powder. It is freely soluble in methanol and dimethyl formamide, slightly soluble in 2-propanol and sparingly soluble in ethanol.
Dabigatran etexilate capsules are supplied in 75 mg, 110 mg and 150 mg strengths for oral administration. Each capsule contains dabigatran etexilate mesylate as the active ingredient: 150 mg dabigatran etexilate (equivalent to 172.95 mg dabigatran etexilate mesylate), 110 mg dabigatran etexilate (equivalent to 126.83 mg dabigatran etexilate mesylate), or 75 mg dabigatran etexilate (equivalent to 86.48 mg dabigatran etexilate mesylate) along with the following inactive ingredients: acacia, dimethicone, hypromellose, hydroxypropyl cellulose, talc, and tartaric acid. The capsule shell is composed of carrageenan, hypromellose, potassium chloride, and titanium dioxide. Black edible ink contains black iron oxide, butyl alcohol, dehydrated alcohol, potassium hydroxide, propylene glycol, and shellac.