Dapsone
Dapsone Prescribing Information
Hypersensitivity to Dapsone and/or its derivatives.
In addition to the warnings listed above, the following syndromes and serious reactions have been reported in patients on Dapsone.
Falsely reduced HbA1c measurements have been reported with dapsone use. Alternate measures of glycemic control (e.g., fructosamine and/or more frequent blood glucose monitoring) are recommended when a discordance between HbA1c and blood glucose concentrations are observed or suspected. Falsely reduced HbA1c may occur without overt evidence of hemolysis or anemia.
Dapsone-USP, 4,4'-diaminodiphenylsulfone (DDS), is a primary treatment for Dermatitis herpetiformis. It is an antibacterial drug for susceptible cases of leprosy. It is a white to yellow crystalline powder. Sparingly soluble in alcohol; Soluble in acetone and in dilute mineral acids; practically insoluble in water.
Dapsone is issued on prescription in tablets of 25 and 100 mg for oral use.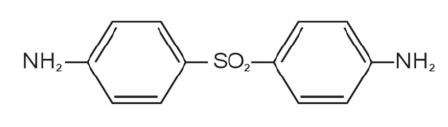
Inactive Ingredients: Colloidal Silicon Dioxide, Corn Starch, Magnesium Stearate and Microcrystalline Cellulose.
USP Dissolution Test Pending.
Dapsone Tablets USP, 25 mg are available as white to creamy white, uncoated round shaped tablets, debossed with “N” above the bisect and “135” below the bisect and plain on other side.
Bottle of 30 tablets NDC 70954-135-10
Bottle of 100 tablets NDC 70954-135-20
Dapsone Tablets USP, 100 mg are available as white to creamy white, uncoated round shaped tablets, debossed with “N” above the bisect and “136” below the bisect and plain on other side.
Bottle of 30 tablets NDC 70954-136-10
Bottle of 100 tablets NDC 70954-136-20