Deferasirox Prescribing Information
- Deferasirox cancause acute renal failure and death, particularly in patients with comorbidities and those who are in the advanced stages of their hematologic disorders.
- Evaluate baseline renal function prior to starting or increasing deferasirox dosing in all patients. Deferasirox is contraindicated in adult and pediatric patients with eGFR less than 40 mL/min/1.73 m2. Measure serum creatinine in duplicate prior to initiation of therapy. Monitor renal function at least monthly. For patients with baseline renal impairment or increased risk of acute renal failure, monitor renal function weekly for the first month, then at least monthly. Reduce the starting dose in patients with preexisting renal disease. During therapy, increase the frequency of monitoring and modify the dose for patients with an increased risk of renal impairment, including use of concomitant nephrotoxic drugs, and pediatric patients with volume depletion or overchelation[see Dosage and Administration , Warnings and Precautions ].
- Deferasiroxcan cause hepatic injury including hepatic failure and death.
- Measure serum transaminases and bilirubin in all patients prior to initiating treatment, every 2 weeks during the first month, and at least monthly thereafter.
- Avoid use of deferasiroxin patients with severe (Child-Pugh C) hepatic impairment and reduce the dose in patients with moderate (Child-Pugh B) hepatic impairment[see.Dosage and Administration,
2.4 Use in Patients with Baseline Hepatic or Renal ImpairmentPatients with Baseline Hepatic ImpairmentMild (Child-Pugh A) Hepatic Impairment: No dose adjustment is necessary.
Moderate (Child-Pugh B) Hepatic Impairment: Reduce the starting dose by 50%.
Severe (Child-Pugh C) Hepatic Impairment: Avoid deferasirox
[see Warnings and Precautions ].Patients with Baseline Renal ImpairmentDo not use deferasirox in adult or pediatric patients with eGFR less than 40 mL/min/1.73 m2
[see Dosage and Administration , Contraindications ].For patients with renal impairment (eGFR 40 to 60 mL/min/1.73 m2), reduce the starting dose by 50%
[see Use in Specific Populations ].Exercise caution in pediatric patients with eGFR between 40 and 60 mL/min/1.73 m2. If treatment is needed, use the minimum effective dose and monitor renal function frequently. Individualize dose titration based on improvement in renal injury
[see Use in Specific Populations ].Warnings and Precautions]5.2 Hepatic Toxicity and FailureDeferasirox can cause hepatic injury, fatal in some patients. In Study 1, 4 patients (1.3%) discontinued deferasirox because of hepatic toxicity (drug-induced hepatitis in 2 patients and increased serum transaminases in 2 additional patients). Hepatic toxicity appears to be more common in patients greater than 55 years of age. Hepatic failure was more common in patients with significant comorbidities, including liver cirrhosis and multi-organ failure
[see Adverse Reactions ]. Acute liver injury and failure, including fatal outcomes, have occurred in pediatric deferasirox-treated patients. Liver failure occurred in association with acute kidney injury in pediatric patients at risk for overchelation during a volume depleting event. Interrupt deferasirox therapy when acute liver injury or acute kidney injury is suspected and during volume depletion.Monitor liver and renal function more frequently in pediatric patients who are receiving deferasirox in the 20 to 40 mg/kg/day range and when iron burden is approaching normal. Use the minimum effective dose to achieve and maintain a low iron burden[see Dosage and Administration , Warnings and Precautions , Adverse Reactions ].Measure transaminases [aspartate transaminase (AST) and alanine transaminase (ALT)] and bilirubin in all patients before the initiation of treatment, and every 2 weeks during the first month and at least monthly thereafter. Consider dose modifications or interruption of treatment for severe or persistent elevations.
Avoid the use of deferasirox in patients with severe (Child-Pugh C) hepatic impairment. Reduce the starting dose in patients with moderate (Child-Pugh B) hepatic impairment
[see Dosage and Administration (2.4), Use in Specific Populations (8.7)]. Patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment may be at higher risk for hepatic toxicity.
- Deferasiroxcan cause gastrointestinal (GI) hemorrhages, which may be fatal, especially in elderly patients who have advanced hematologic malignancies and/or low platelet counts.
- Monitor patients and discontinue deferasiroxfor suspected GI ulceration or hemorrhage[see.Warnings and Precautions]
5.3 Gastrointestinal (GI) Ulceration, Hemorrhage, and
PerforationGI hemorrhage, including deaths, has been reported in deferasirox-treated patients, especially in elderly patients who had advanced hematologic malignancies and/or low platelet counts. Nonfatal upper GI irritation, ulceration and hemorrhage have been reported in patients, including children and adolescents, receiving deferasirox
[see Adverse Reactions ]. Monitor for signs and symptoms of GI ulceration and hemorrhage during deferasirox therapy and promptly initiate additional evaluation and treatment if a serious GI adverse reaction is suspected. The risk of GI hemorrhage may be increased when administering deferasirox in combination with drugs that have ulcerogenic or hemorrhagic potential, such as nonsteroidal anti-inflammatory drugs (NSAIDs), corticosteroids, oral bisphosphonates, or anticoagulants. There have been reports of ulcers complicated with GI perforation (including fatal outcome)[see Adverse Reactions ].
Deferasirox tablets for oral suspension are available as follows:
White to off-white, round, unscored tablets, debossed with  and 454 on one side and plain on the other side.
and 454 on one side and plain on the other side.
White to off-white, round, unscored tablets, debossed with  and 455 on one side and plain on the other side.
and 455 on one side and plain on the other side.
White to off-white, round, unscored tablets, debossed with  and 456 on one side and plain on the other side.
and 456 on one side and plain on the other side.
Counsel patients to use non-hormonal method(s) of contraception since deferasirox can render hormonal contraceptives ineffective
Deferasirox is contraindicated in patients with:
- Estimated GFR less than 40 mL/min/1.73 m2 [see Dosage and Administration , Warnings and Precautions ];
- Poor performance status; [see Warnings and Precautions ]
- High-risk myelodysplastic syndromes; (this patient population was not studied and is not expected to benefit from chelation therapy)
- Advanced malignancies;[see Warnings and Precautions ]
- Platelet counts less than 50 x 109/L;[see Warnings and Precautions , Adverse Reactions ].
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Acute Kidney Injury, Including Acute Renal Failure Requiring Dialysis, and Renal Tubular Toxicity Including Fanconi Syndrome[see Warnings and Precautions ]
- Hepatic Toxicity and Failure [see Warnings and Precautions ]
- Hypersensitivity [see Warnings and Precautions ]
- Severe Skin Reactions [see Warnings and Precautions ]
- Skin Rash [see Warnings and Precautions ]
- Auditory and Ocular Abnormalities [see Warnings and Precautions ( 5.10)]
Deferasirox is an iron chelating agent. Deferasirox Tablets For Oral Suspension contain 125 mg, 250 mg, or 500 mg of deferasirox. Deferasirox is designated chemically as 4-[3,5-Bis (2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]-benzoic acid and its structural formula is:
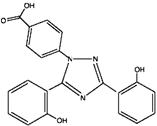
C21H15N3O4 M.W. 373.4
Deferasirox is an off-white to pale yellow powder.