Deferasirox Prescribing Information
- Deferasirox can cause acute renal failure and death, particularly in patients with comorbidities and those who are in the advanced stages of their hematologic disorders.
- Evaluate baseline renal function prior to starting or increasing deferasirox dosing in all patients. Deferasirox is contraindicated in adult and pediatric patients with eGFR less than 40 mL/min/1.73 m2. Measure serum creatinine in duplicate prior to initiation of therapy. Monitor renal function at least monthly. For patients with baseline renal impairment or increased risk of acute renal failure, monitor renal function weekly for the first month, then at least monthly. Reduce the starting dose in patients with preexisting renal disease. During therapy, increase the frequency of monitoring and modify the dose for patients with an increased risk of renal impairment, including use of concomitant nephrotoxic drugs, and pediatric patients with volume depletion or overchelation[see Dosage and Administration , Warnings and Precautions , Adverse Reactions ].
- Deferasirox can cause hepatic injury including hepatic failure and death.
- Measure serum transaminases and bilirubin in all patients prior to initiating treatment, every 2 weeks during the first month, and at least monthly thereafter.
- Avoid use of deferasirox in patients with severe (Child-Pugh C) hepatic impairment and reduce the dose in patients with moderate (Child-Pugh B) hepatic impairment[see Dosage and Administration , Warnings and Precautions ].
- Deferasirox can cause gastrointestinal (GI) hemorrhages, which may be fatal, especially in elderly patients who have advanced hematologic malignancies and/or low platelet counts.
- Monitor patients and discontinue deferasirox for suspected GI ulceration or hemorrhage[see Warnings and Precautions ].
- 125 mg tablets
White to off white, round, flat tablet with beveled edges, debossed with "TEVA" on one side and "7011" on the other side.
- 250 mg tablets
White to off white, round, flat tablet with beveled edges, debossed with "TEVA" on one side and "7012" on the other side.
- 500 mg tablets
White to off white, round, flat tablet with beveled edges, debossed with "TEVA" on one side and "7013" on the other side.
Deferasirox is contraindicated in patients with:
- Estimated GFR less than 40 mL/min/1.73 m2 [see Dosage and Administration , Warnings and Precautions ];
- Poor performance status; [see Warnings and Precautions (5.1, 5.3)]
- High-risk myelodysplastic syndromes; (this patient population was not studied and is not expected to benefit from chelation therapy)
- Advanced malignancies. [see Warnings and Precautions (5.1,5.3)]
- Platelet counts less than 50 x 109/L [see Warnings and Precautions (5.3, 5.4)
- Known hypersensitivity to deferasirox or any component of deferasirox tablets for oral suspension [see Warnings and Precautions , Adverse Reactions ].
The following clinically significant adverse reactions are also discussed in other sections of the labeling:
- Acute Kidney Injury, Including Acute Renal Failure Requiring Dialysis, and Renal Tubular Toxicity Including Fanconi Syndrome [see Warnings and Precautions ]
- Hepatic Toxicity and Failure [see Warnings and Precautions ]
- GI Hemorrhage [see Warnings and Precautions ]
- Bone Marrow Suppression [see Warnings and Precautions ]
- Hypersensitivity [see Warnings and Precautions ]
- Severe Skin Reactions [see Warnings and Precautions ]
- Skin Rash [see Warnings and Precautions ]
- Auditory and Ocular Abnormalities [see Warnings and Precautions ]
Deferasirox is an iron chelating agent. Deferasirox tablets for oral suspension contain 125 mg, 250 mg, or 500 mg deferasirox. Deferasirox is designated chemically as 4-[3,5-Bis(2-hydroxyphenyl)-1H-1,2,4-triazol-1-yl]-benzoic acid and its structural formula is:
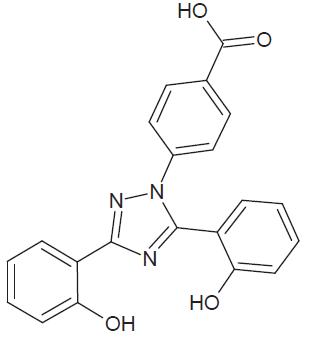
C21H15N3O4 M.W. 373.36
Deferasirox is a white to almost white powder.
Deferasirox is an orally active chelator that is selective for iron (as Fe3+). It is a tridentate ligand that binds iron with high affinity in a 2:1 ratio. Although deferasirox has very low affinity for zinc and copper there are variable decreases in the serum concentration of these trace metals after the administration of deferasirox. The clinical significance of these decreases is uncertain.