Deflazacort
Deflazacort Prescribing Information
Deflazacort tablets are indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients 5 years of age and older.
TM(deflazacort) tablets. However, due to PTC Therapeutics, Inc.'s marketing exclusivity rights, this drug product is not labeled with that information.
- 6 mg: White to off-white round tablet with “C1” debossed on one side
- 18 mg: White to off-white round tablet with “C2” debossed on one side
- 30 mg: White to off-white oval tablet with “C3” debossed on one side
- 36 mg: White to off-white oval tablet with “C4” debossed on one side
Deflazacort tablets are contraindicated in patients with known hypersensitivity to deflazacort or to any of the inactive ingredients. Instances of hypersensitivity, including anaphylaxis, have occurred in patients receiving corticosteroid therapy
The following serious adverse reactions are discussed in more detail in other sections:
- Alterations in Endocrine Function[see Warnings and Precautions (5.1)]
- Immunosuppression and Increased Risk of Infection[see Warnings and Precautions (5.2)]
- Alterations in Cardiovascular/Renal Function[see Warnings and Precautions (5.3)]
- Gastrointestinal Perforation[see Warnings and Precautions (5.4)]
- Behavioral and Mood Disturbances[see Warnings and Precautions (5.5)]
- Effects on Bones[see Warnings and Precautions (5.6)]
- Ophthalmic Effects[see Warnings and Precautions (5.7)]
- Immunizations[see Warnings and Precautions (5.8)]
- Serious Skin Rashes[see Warnings and Precautions (5.9)]
- Effects on Growth and Development[see Warnings and Precautions (5.10)]
- Myopathy[see Warnings and Precautions (5.11)]
- Kaposi’s Sarcoma[see Warnings and Precautions (5.12)]
- Thromboembolic Events[see Warnings and Precautions (5.14)]
- Anaphylaxis[see Warnings and Precautions (5.15)]
The active ingredient in deflazacort tablets is deflazacort (a corticosteroid). Corticosteroids are adrenocortical steroids, both naturally occurring and synthetic. The molecular formula for deflazacort is C
25H
31NO
6. The chemical name for deflazacort is 16α,17-isoxazole-11β,21-dihydroxypregna-1,4-diene-3,20-dione 21acetate, and the structure is:
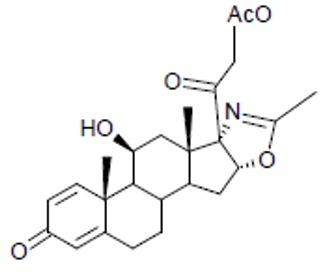
Deflazacort is a white or almost white crystalline powder and has a molecular weight of 441.52. Deflazacort is soluble in dichloromethane and chloroform, slightly soluble in ethanol and ethyl acetate, almost insoluble in water.
Deflazacort tablets for oral administration are available as an immediate-release tablet in strengths of 6, 18, 30 and 36 mg. Each tablet contains deflazacort and the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, and pregelatinized starch (maize).
The effectiveness of deflazacort for the treatment of DMD was established in Study 1, a multicenter, randomized, double-blind, placebo-controlled, 52-week study conducted in the US and Canada. The study population consisted of 196 male pediatric patients 5 to 15 years of age with documented mutation of the dystrophin gene, onset of weakness before 5 years of age, and serum creatinine kinase activity at least 10 times the upper limit of normal (ULN) at some stage in their illness. Patients were randomized to therapy with deflazacort (0.9 or 1.2 mg/kg/day), an active comparator, or placebo. A comparison to placebo was made after 12 weeks of treatment. After 12 weeks, placebo patients were re-randomized to receive either deflazacort or the active comparator; all patients continued treatment for an additional 40 weeks. Baseline characteristics were comparable between the treatment arms.
In Study 1, efficacy was evaluated by assessing the change between Baseline and Week 12 in average strength of 18 muscle groups. Individual muscle strength was graded using a modified Medical Research Council (MRC) 11-point scale, with higher scores representing greater strength.
The change in average muscle strength score between Baseline and Week 12 was significantly greater for the deflazacort 0.9 mg/kg/day dose group than for the placebo group (see Table 2).
Treatment | N | Change from Baseline LS Mean (95% CI) | P-value |
Deflazacort 0.9 mg/kg/day | 51 | 0.15 (0.01, 0.28) | 0.017 |
Placebo | 50 | -0.10 (-0.23, 0.03) |
Compared with the deflazacort 0.9 mg/kg/day group, the deflazacort 1.2 mg/kg/day group demonstrated a small additional benefit compared to placebo at Week 12, but had a greater incidence of adverse reactions. Therefore, use of a 1.2 mg/kg/day dosage of deflazacort is not recommended
Although not a pre-specified statistical analysis, compared with placebo, the deflazacort 0.9 mg/kg/day dose group demonstrated at Week 52 the persistence of the treatment effect observed at Week 12 and the small advantage of the 1.2 mg/kg/day dose that was observed at Week 12 was no longer present. Also not statistically controlled for multiple comparisons, results on several timed measures of patient function (i.e., time to stand from supine, time to climb 4 stairs, and time to walk or run 30 feet) numerically favored deflazacort 0.9 mg/kg/day at Week 12, in comparison with placebo.
An additional randomized, double-blind, placebo-controlled, 104-week clinical trial evaluated deflazacort in comparison to placebo (Study 2). The study population consisted of 29 male children 6 to 12 years of age with a DMD diagnosis confirmed by the documented presence of abnormal dystrophin or a confirmed mutation of the dystrophin gene. The results of the analysis of the primary endpoint of average muscle strength scores in Study 2 (graded on a 0 to 5 scale) at 2 years were not statistically significant, possibly because of a limited number of patients remaining in the placebo arm (subjects were discontinued from the trial when they lost ambulation). Although not statistically controlled for multiple comparisons, average muscle strength scores at Months 6 and 12, as well as the average time to loss of ambulation, numerically favored deflazacort in comparison with placebo.