Dexamethasone Sodium Phosphate
Dexamethasone Sodium Phosphate Prescribing Information
Acute adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; mineralocorticoid supplementation may be necessary, particularly when synthetic analogs are used).
Preoperatively, and in the event of serious trauma or illness, in patients with known adrenal insufficiency or when adrenocortical reserve is doubtful.
Shock unresponsive to conventional therapy if adrenocortical insufficiency exists or is suspected.
Congenital adrenal hyperplasia.
Nonsuppurative thyroiditis.
Hypercalcemia associated with cancer.
Post-traumatic osteoarthritis.
Synovitis of osteoarthritis.
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy).
Acute and subacute bursitis.
Epicondylitis.
Acute nonspecific tenosynovitis.
Acute gouty arthritis.
Psoriatic arthritis.
Ankylosing spondylitis.
Systemic lupus erythematosus.
Acute rheumatic carditis.
Pemphigus.
Severe erythema multiforme (Stevens-Johnson Syndrome).
Exfoliative dermatitis.
Bullous dermatitis herpetiformis.
Severe seborrheic dermatitis.
Severe psoriasis.
Mycosis fungoides.
Bronchial asthma.
Contact dermatitis.
Atopic dermatitis.
Serum sickness.
Seasonal or perennial allergic rhinitis.
Drug hypersensitivity reactions.
Urticarial transfusion reactions.
Acute noninfectious laryngeal edema (epinephrine is the drug of first
choice).
Herpes zoster ophthalmicus.
Iritis, iridocyclitis.
Chorioretinitis.
Diffuse posterior uveitis and choroiditis.
Optic neuritis.
Sympathetic ophthalmia.
Anterior segment inflammation.
Allergic conjunctivitis.
Allergic corneal marginal ulcers.
Keratitis.
Ulcerative colitis (systemic therapy).
Regional enteritis (systemic therapy).
Symptomatic Sarcoidosis.
Berylliosis.
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate anti-tuberculosis chemotherapy.
Loeffler's syndrome not manageable by other means.
Aspiration pneumonitis.
Acquired (autoimmune) hemolytic anemia.
Idiopathic thrombocytopenic purpura in adults (I.V. only; I.M. administration is contraindicated).
Secondary thrombocytopenia in adults.
Erythroblastopenia (RBC anemia).
Congenital (erythroid) hypoplastic anemia.
Leukemias and lymphomas in adults.
Acute leukemia of childhood.
Acute exacerbations of multiple sclerosis.
Tuberculous meningitis with subarachnoid block or impending block when used concurrently with appropriate anti-tuberculosis chemotherapy.
Trichinosis with neurologic or myocardial involvement.
Diagnostic testing of adrenocortical hyperfunction.
Cerebral edema of diverse etiologies in conjunction with adequate neurological evaluation and management.
Synovitis of osteoarthritis.
Rheumatoid arthritis.
Acute and subacute bursitis.
Acute gouty arthritis.
Epicondylitis.
Acute nonspecific tenosynovitis.
Post-traumatic osteoarthritis.
Keloids.
Localized hypertrophic, infiltrated, inflammatory lesions of:
lichen planus, psoriatic plaques, granuloma annulare, and lichen simplex chronicus (neurodermatitis).
Discoid lupus erythematosus.
Necrobiosis lipoidica diabeticorum.
Alopecia areata.
They also may be useful in cystic tumors of an aponeurosis tendon (ganglia).
For the treatment of unresponsive shock high pharmacologic doses of this product are currently recommended. Reported regimens range from 1 mg/kg to 6 mg/kg of body weight as a single intravenous injection to 40 mg initially followed by repeat intravenous injection every 2 to 6 hours while shock persists.
For the treatment of cerebral edema in adults an initial intravenous dose of 10 mg is recommended followed by 4 mg intramuscularly every six hours until maximum response has been noted. This regimen may be continued for several days postoperatively in patients requiring brain surgery. Oral dexamethasone, 1 mg to 3 mg t.i.d., should be given as soon as possible and dosage tapered off over a period of five to seven days. Non-operative cases may require continuous therapy to remain free of symptoms of increased intracranial pressure. The smallest effective dose should be used in children, preferably orally. This may approximate 0.2 mg/kg/24 hours in divided doses.
In treatment of acute exacerbations of multiple sclerosis daily doses of 200 mg of prednisolone for a week followed by 80 mg every other day or 4 mg to 8 mg dexamethasone every other day for 1 month have been shown to be effective.
The initial dosage should be maintained or adjusted until a satisfactory response is noted. If after a reasonable period of time there is a lack of satisfactory clinical response, dexamethasone sodium phosphate injection should be discontinued and the patient transferred to other appropriate therapy. It should be emphasized that dosage requirements are variable and must be individualized on the basis of the disease under treatment and the response of the patient.
After a favorable response is noted, the proper maintenance dosage should be determined by decreasing the initial drug dosage in small decrements at appropriate time intervals until the lowest dosage which will maintain an adequate clinical response is reached. It should be kept in mind that constant monitoring is needed in regard to drug dosage. Included in the situations which may make dosage adjustments necessary are changes in clinical status secondary to remissions or exacerbations in the disease process, the patient’s individual drug responsiveness and the effect of patient exposure to stressful situations not directly related to the disease entity under treatment. In this later situation it may be necessary to increase the dosage of dexamethasone sodium phosphate injection for a period of time consistent with the patient’s condition. If after a long-term therapy the drug is to be stopped, it is recommended that it be withdrawn gradually rather than abruptly.
Intrasynovial and soft tissue injections should be employed only when affected areas are limited to 1 or 2 sites. It should be remembered that corticoids provide palliation only and that other conventional or curative methods of therapy should be employed when indicated.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Frequency of injection usually ranges from once every 3 to 5 days to once every 2 to 3 weeks. Frequent intra-articular injection may cause damage to joint tissue.
Systemic fungal infections.
Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss
Hypokalemic alkalosis
Hypertension
Muscle weakness
Steroid myopathy
Loss of muscle mass
Osteoporosis
Vertebral compression fractures
Aseptic necrosis of femoral and humeral heads
Pathologic fracture of long bones
Peptic ulcer with possible subsequent perforation and hemorrhage
Pancreatitis
Abdominal distention
Ulcerative esophagitis
Impaired wound healing
Thin fragile skin
Facial erythema
Increased sweating
May suppress reactions to skin tests
Petechiae and ecchymoses
Convulsions
Increased intracranial pressure with papilledema (pseudotumor cerebri) usually after treatment
Vertigo
Headache
Posterior subcapsular cataracts
Increased intraocular pressure
Glaucoma
Menstrual irregularities
Development of cushingoid state
Suppression of growth in children
Secondary adrenocortical and pituitary unresponsiveness, particularly in times of stress, as in trauma, surgery, or illness
Decreased carbohydrate tolerance
Manifestations of latent diabetes mellitus
Increased requirements for insulin or oral hypoglycemic agents in diabetics
Negative nitrogen balance due to protein catabolism
Hyperpigmentation or hypopigmentation
Subcutaneous and cutaneous atrophy
Sterile abscess
Postinjection flare, following intra-articular use
Charcot-like arthropathy
Itching, burning, tingling in the ano-genital region
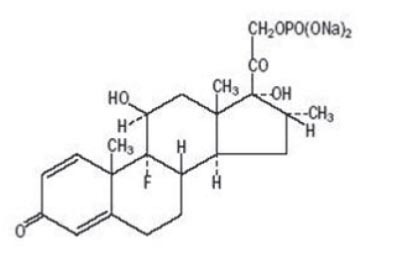
Dexamethasone sodium phosphate injection, USP is a water-soluble inorganic ester of dexamethasone which produces a rapid response even when injected intramuscularly.
Dexamethasone sodium phosphate, C22H28FNa2O8P, has a molecular weight of 516.41 and chemically is Pregna-1,4-diene-3,20-dione, 9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-, disodium salt, (11β,16α).
It occurs as a white to off-white powder, is exceedingly hygroscopic, is soluble in water and its solutions have a pH between 7.5 and 10.5. It has the following structural formula:
Dexamethasone sodium phosphate injection, USP is available in 4 mg/mL concentration.
Each mL of dexamethasone sodium phosphate injection USP, 4 mg/mL, contains 4.37 mg dexamethasone sodium phosphate, USP equivalent to 4 mg dexamethasone phosphate; 1 mg sodium sulfite; 10 mg benzyl alcohol (preservative); and water for injection (q.s.). Made isotonic with sodium citrate. pH adjusted with citric acid or sodium hydroxide.
Dexamethasone sodium phosphate injection USP,
1 mL, 25 Single-Dose Vials in a Carton: NDC 70121-1450-5
5 mL, 25 Multiple-Dose Vials in a Carton: NDC 70121-1451-5
Discard unused portion of single-dose vials.
Manufactured by:
Distributed by:
Rev. 02-2024-03