Dexamethasone Sodium Phosphate
Dexamethasone Sodium Phosphate Prescribing Information
Primary or secondary adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; synthetic analogs may be used in conjunction with mineralocorticoids where applicable; in infancy, mineralocorticoid supplementation is of particular importance).
Acute adrenocortical insufficiency (hydrocortisone or cortisone is the drug of choice; mineralocorticoid supplementation may be necessary, particularly when synthetic analogs are used).
Preoperatively, and in the event of serious trauma or illness, in patients with known adrenal insufficiency or when adrenocortical reserve is doubtful.
Shock unresponsive to conventional therapy if adrenocortical insufficiency exists or is suspected.
Congenital adrenal hyperplasia.
Nonsuppurative thyroiditis.
Hypercalcemia associated with cancer.
As adjunctive therapy for short-term administration (to tide the patient over an acute episode or exacerbation) in:
Post-traumatic osteoarthritis.
Synovitis of osteoarthritis.
Rheumatoid arthritis, including juvenile rheumatoid arthritis (selected cases may require low-dose maintenance therapy).
Acute and subacute bursitis.
Epicondylitis.
Acute nonspecific tenosynovitis.
Acute gouty arthritis.
Psoriatic arthritis.
Ankylosing spondylitis.
During an exacerbation or as maintenance therapy in selected cases of:
Systemic lupus erythematosus.
Acute rheumatic carditis.
Pemphigus.
Severe erythema multiforme. (Stevens-Johnson Syndrome)
Exfoliative dermatitis.
Bullous dermatitis herpetiformis.
Severe seborrheic dermatitis.
Severe psoriasis.
Mycosis fungoides.
Control of severe or incapacitating allergic conditions intractable to adequate trials of conventional treatment in:
Bronchial asthma.
Contact dermatitis.
Atopic dermatitis.
Serum sickness.
Seasonal or perennial allergic rhinitis.
Drug hypersensitivity reactions.
Urticarial transfusion reactions.
Acute noninfectious laryngeal edema (epinephrine is the drug of first choice).
Severe acute and chronic allergic and inflammatory processes involving the eye, such as:
Herpes zoster ophthalmicus.
Iritis, iridocyclitis.
Chorioretinitis.
Diffuse posterior uveitis and choroiditis.
Optic neuritis.
Sympathetic ophthalmia.
Anterior segment inflammation.
Allergic conjunctivitis.
Keratitis.
Allergic corneal marginal ulcers.
To tide the patient over a critical period of the disease in:
Ulcerative colitis (systemic therapy).
Regional enteritis (systemic therapy).
Symptomatic sarcoidosis.
Berylliosis.
Fulminating or disseminated pulmonary tuberculosis when used concurrently with appropriate antituberculous chemotherapy.
Loeffler's syndrome not manageable by other means.
Aspiration pneumonitis.
Acquired (autoimmune) hemolytic anemia.
Idiopathic thrombocytopenic purpura in adults (IV only; IM administration is contraindicated).
Secondary thrombocytopenia in adults.
Erythroblastopenia (RBC anemia).
Congenital (erythroid) hypoplastic anemia.
For palliative management of:
Leukemias and lymphomas in adults.
Acute leukemia of childhood.
To induce diuresis or remission of proteinuria in the nephrotic syndrome, without uremia, of the idiopathic type or that due to lupus erythematosus.
Tuberculosis meningitis with subarachnoid block or impending block when used concurrently with appropriate antituberculous chemotherapy.
Trichinosis with neurologic or myocardial involvement.
Dexamethasone sodium phosphate injection, USP 4 mg/mL is for intravenous, intramuscular, intra-articular, intralesional and soft tissue injection.
Dexamethasone sodium phosphate injection, USP 10 mg/mL is for intravenous or intramuscular use only.
Dexamethasone sodium phosphate injection, USP can be given directly from the vial, or it can be added to sodium chloride injection or dextrose injection and administered by intravenous drip.
Solutions used for intravenous administration or further dilution of this product should be preservative-free when used in the neonate, especially the premature infant.
When it is mixed with an infusion solution, sterile precautions should be observed. Since infusion solutions generally do not contain preservatives, mixtures should be used within 24 hours.
Systemic fungal infections. (See
Serious neurologic events, some resulting in death, have been reported with epidural injection of corticosteroids. Specific events reported include, but are not limited to, spinal cord infarction, paraplegia, quadriplegia, cortical blindness, and stroke. These serious neurologic events have been reported with and without use of fluoroscopy. The safety and effectiveness of epidural administration of corticosteroids has not been established, and corticosteroids are not approved for this use.
In patients on corticosteroid therapy subject to any unusual stress, increased dosage of rapidly acting corticosteroids before, during and after the stressful situation is indicated.
Corticosteroids, including dexamethasone sodium phosphate injection, (suppress the immune systemand increase the risk of infection with any pathogen, including viral, bacterial,fungal, protozoan, or helminthic pathogens. Corticosteroids can:
• Reduce resistance to new infections• Exacerbate existing infections• Increase the risk of disseminated infections• Increase the risk of reactivation or exacerbation of latent infections• Mask some signs of infection
Corticosteroid-associated infections can be mild but can be severe and at times fatal. The rate of infectious complications increases with increasing corticosteroid dosages.
Monitor for the development of infection and consider dexamethasone sodium phosphate injection withdrawal or dosage reduction as needed.
If dexamethasone sodium phosphate injection is used to treat a condition in patients with latent tuberculosis or tuberculin reactivity, tuberculosis may occur. Closely monitor such patients for reactivation. During prolonged therapy, patients with latent tuberculosis or tuberculin reactivity should receive chemoprophylaxis.
Varicella and measles can have a serious or even fatal course in non-immune patients taking corticosteroids, including dexamethasone sodium phosphate injection. In corticosteroid-treated patients who have not had these diseases or are nonimmune, particular care should be taken to avoid exposure to varicella and measles:
• If a dexamethasone sodium phosphate injection -treated patient is exposed to varicella, prophylaxis with varicella zoster immune globulin may be indicated. If varicella develops, treatment with antiviral agents may be considered.• If a dexamethasone sodium phosphate injection - treated patient is exposed to measles, prophylaxis with immunoglobulin may be indicated.
Hepatitis B virus reactivation can occur in patients who are hepatitis B carriers treated with immunosuppressive dosages of corticosteroids, including dexamethasone sodium phosphate injection. Reactivation can also occur infrequently in corticosteroid-treated patients who appear to have resolved hepatitis B infection. Screen patients for hepatitis B infection before initiating immunosuppressive (e.g., prolonged) treatment with dexamethasone sodium phosphate injection. For patients who show evidence of hepatitis B infection, recommend consultation with physicians with expertise in managing hepatitis B regarding monitoring and consideration for hepatitis B antiviral therapy.
Corticosteroids, including dexamethasone sodium phosphate injection, may exacerbate systemic fungal infections; therefore, avoid dexamethasone sodium phosphate injection use in the presence of such infections unless dexamethasone sodium phosphate injection is needed to control drug reactions. For patients on chronic dexamethasone sodium phosphate injection therapy who develop systemic fungal infections, dexamethasone sodium phosphate injection withdrawal or dosage reduction is recommended.
Corticosteroids, including dexamethasone sodium phosphate injection, may activate latent amebiasis. Therefore, it is recommended that latent amebiasis or active amebiasis be ruled out before initiating dexamethasone sodium phosphate injection in patients who have spent time in the tropics or patients with unexplained diarrhea.
Corticosteroids, including dexamethasone sodium phosphate injection, should be used with great care in patients with known or suspected Strongyloides (threadworm) infestation. In such patients, corticosteroid-induced immunosuppression may lead to Strongyloides hyperinfection and dissemination with widespread larval migration, often accompanied by severe enterocolitis and potentially fatal gram-negative septicemia.
Avoid corticosteroids, including dexamethasone sodium phosphate injection, in patients with cerebral malaria.
Kaposi’s sarcoma has been reported to occur in patients receiving corticosteroid therapy, most often for chronic conditions. Discontinuation of corticosteroids may result in clinical improvement of Kaposi’s sarcoma.
Because rare instances of anaphylactoid reactions have occurred in patients receiving parenteral corticosteroid therapy, appropriate precautionary measures should be taken prior to administration, especially when the patient has a history of allergy to any drug. Anaphylactoid and hypersensitivity reactions have been reported for dexamethasone sodium phosphate injection. (See
Corticosteroids may exacerbate systemic fungal infections and, therefore, should not be used in the presence of such infections unless they are needed to control drug reactions due to amphotericin B. Moreover, there have been cases reported in which concomitant use of amphotericin B and hydrocortisone was followed by cardiac enlargement and congestive failure.
In patients on corticosteroid therapy subjected to any unusual stress, increased dosage of rapidly acting corticosteroids before, during, and after the stressful situation is indicated.
Drug-induced secondary adrenocortical insufficiency may result from too rapid withdrawal of corticosteroids and may be minimized by gradual reduction of dosage. This type of relative insufficiency may persist for months after discontinuation of therapy; therefore, in any situation of stress occurring during that period, hormone therapy should be reinstituted. If the patient is receiving steroids already, dosage may have to be increased. Since mineralocorticoid secretion may be impaired, salt and/or a mineralocorticoid should be administered concurrently.
Moreover, corticosteroids may affect the nitroblue-tetrazolium test for bacterial infection and produce false negative results.
In cerebral malaria, a double-blind trial has shown that the use of corticosteroids is associated with prolongation of coma and a higher incidence of pneumonia and gastrointestinal bleeding.
Prolonged use of corticosteroids may produce posterior subcapsular cataracts, glaucoma with possible damage to the optic nerves, and may enhance the establishment of secondary ocular infections due to fungi or viruses.
Average and large doses of cortisone or hydrocortisone can cause elevation of blood pressure, salt and water retention, and increased excretion of potassium. These effects are less likely to occur with the synthetic derivatives except when used in large doses. Dietary salt restriction and potassium supplementation may be necessary. All corticosteroids increase calcium excretion.
Administration of live virus vaccines, including smallpox, is contraindicated in individuals receiving immunosuppressive doses of corticosteroids. If inactivated viral or bacterial vaccines are administered to individuals receiving immunosuppressive doses of corticosteroids, the expected serum antibody response may not be obtained. However, immunization procedures may be undertaken in patients who are receiving corticosteroids as replacement therapy, e.g., for Addison's disease.
Literature reports suggest an apparent association between use of corticosteroids and left ventricular free wall rupture after a recent myocardial infarction; therefore, therapy with corticosteroids should be used with great caution in these patients.
Since adequate human reproduction studies have not been done with corticosteroids, use of these drugs in pregnancy or in women of childbearing potential requires that the anticipated benefits be weighed against the possible hazards to the mother and embryo or fetus. Infants born of mothers who have received substantial doses of corticosteroids during pregnancy should be carefully observed for signs of hypoadrenalism.
Corticosteroids appear in breast milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other unwanted effects. Mothers taking pharmacologic doses of corticosteroids should be advised not to nurse.
Sodium retention
Fluid retention
Congestive heart failure in susceptible patients
Potassium loss
Hypokalemic alkalosis
Hypertension
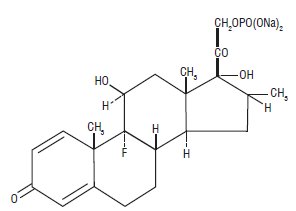
Dexamethasone sodium phosphate, a synthetic adrenocortical steroid, is a white or slightly yellow, crystalline powder. It is freely soluble in water and is exceedingly hygroscopic. The molecular weight is 516.41. It is designated chemically as 9-fluoro-11β,17-dihydroxy-16α-methyl-21-(phosphonooxy)pregna-1,4-diene-3,20-dione disodium salt. The empirical formula is C22H28FNa2O8P and the structural formula is:
Dexamethasone sodium phosphate injection, USP is a sterile solution of dexamethasone sodium phosphate, and is supplied in 4 mg/ mL and 10 mg /mL.
Dexamethasone sodium phosphate injection, USP 4 mg/mL is a sterile solution for intravenous, intramuscular, intra-articular, intralesional and soft tissue administration. Each mL contains:
Dexamethasone sodium phosphate injection, USP 10 mg/mL is a sterile solution for intravenous or intramuscular use only. Each mL contains:
Dexamethasone sodium phosphate injection has a rapid onset but short duration of action when compared with less soluble preparations. Because of this, it is suitable for the treatment of acute disorders responsive to adrenocortical steroid therapy.
Naturally occurring glucocorticoids (hydrocortisone and cortisone), which also have salt-retaining properties, are used as replacement therapy in adrenocortical deficiency states. Their synthetic analogs, including dexamethasone, are primarily used for their potent anti-inflammatory effects in disorders of many organ systems.
Glucocorticoids cause profound and varied metabolic effects. In addition, they modify the body's immune responses to diverse stimuli.
At equipotent anti-inflammatory doses, dexamethasone almost completely lacks the sodium-retaining property of hydrocortisone and closely related derivatives of hydrocortisone.