Dextrose
Dextrose Prescribing Information
70% Dextrose Injection USP is indicated as a caloric component in a parenteral nutrition regimen. 70% Dextrose Injection USP is used with an appropriate protein (nitrogen) source in the prevention of nitrogen loss or in the treatment of negative nitrogen balance in patients where: (1) the alimentary tract cannot or should not be used, (2) gastrointestinal absorption of protein is impaired, or (3) metabolic requirements for protein are substantially increased, as with extensive burns.
This solution is for intravenous use only after admixture or dilution.
70% Dextrose Injection USP is designed for use with automated compounding devices for preparing intravenous nutritional admixtures or for the filling of empty sterile syringes. Dosages will be in accordance with the recommendation of the prescribing physician. 70% Dextrose Injection USP is not intended for direct infusion. Admixtures should be made by, or under the direction of, a pharmacist using strict aseptic technique under a laminar flow hood. Compounded admixtures may be stored under refrigeration for up to 24 hours. Administration of admixtures should be completed within 24 hours after removal from refrigeration.
Dosage is to be directed by a physician and is dependent upon age, weight, clinical condition of the patient and laboratory determinations. Frequent laboratory determinations and clinical evaluation are essential to monitor changes in blood glucose and electrolyte concentrations, and fluid and electrolyte balance during prolonged parenteral therapy.
Fluid Administration should be based on calculated maintenance or replacement fluid requirements for each patient.
The infusion of 70% Dextrose Injection USP is contraindicated in patients having intracranial or intraspinal hemorrhage, in patients who are severely dehydrated, in patients who are anuric, and in patients in hepatic coma.
Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia. Incompatibility of admixed components can produce precipitates which may cause particulate emboli.
Hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause hypovolemia, dehydration, mental confusion and/or loss of consciousness. Too rapid infusion of hypertonic solutions may cause local pain and venous irritation. Rate of administration should be adjusted according to tolerance. Use of the largest peripheral vein and a small bore needle is recommended. (See
Hypersensitivity reactions, including anaphylaxis and chills.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures, and save the remainder of the fluid for examination if deemed necessary.
Caution must be exercised in the administration of 70% Dextrose Injection USP to patients receiving corticosteroids or corticotropin. Some additives may be incompatible. Consult with pharmacist. When introducing additives, use aseptic techniques. Mix thoroughly. Do not store. Dispose of any unused product. See
Dilute before use to a concentration which will, when administered with an amino acid (nitrogen) source, result in an appropriate calorie to gram of nitrogen ratio and which has an osmolarity consistent with the route of administration.
Unless appropriately diluted, the infusion of hypertonic dextrose injection into a peripheral vein may result in vein irritation, vein damage, and thrombosis. Strongly hypertonic nutrient solutions should only be administered through an indwelling intravenous catheter with the tip located in a large central vein such as the superior vena cava.
Use of 70% Dextrose Injection USP to prepare parenteral nutritional admixtures may be incompatible with other components, especially calcium and phosphate salts and lipid emulsions. Incompatibility of admixed components can produce precipitates which may cause particulate emboli. Use 70% Dextrose Injection USP only to prepare formulations that are known to be stable: refer to standard texts for further information.
The administration of intravenous solutions can cause fluid and/or solute overload resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema. The risk of dilutional states is inversely proportional to the electrolyte concentration.
WARNING: 70% Dextrose Injection USP contains aluminum that may be toxic. Aluminum may reach toxic levels with prolonged parenteral administration if kidney function is impaired. Premature neonates are particularly at risk because their kidneys are immature, and they require large amounts of calcium and phosphate solutions, which contain aluminum.
Research indicates that patients with impaired kidney function, including premature neonates, who receive parenteral levels of aluminum at greater than 4 to 5 mcg/kg/day accumulate aluminum at levels associated with central nervous system and bone toxicity. Tissue loading may occur at even lower rates of administration.
Prolonged infusion of isotonic or hypotonic dextrose in water may increase the volume of extracellular fluid and cause water intoxication.
Solutions containing dextrose without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility of agglomeration.
Excessive administration of potassium-free dextrose solutions may result in significant hypokalemia. Serum potassium levels should be maintained and potassium supplemented as required.
In very low birth weight infants, excessive or rapid administration of dextrose injection may result in increased serum osmolality and possible intracerebral hemorrhage.
Each 100 mL of
Hydrous Dextrose USP 70 g; Water for Injection USP qs
pH: 4.0 (3.2-6.5); Calculated Osmolarity: 3532 mOsmol/liter, hypertonic.
Specific gravity 1.233 at 25°C
Calories per liter: 2380 calculated on the basis of 3.4 kcal/g of dextrose, hydrous
70% Dextrose Injection USP is sterile, nonpyrogenic and contains no bacteriostatic or antimicrobial agents. This product is intended for intravenous administration after appropriate admixture or dilution as a caloric source.
The formula of the active ingredient is:
| Ingredient | Molecular Formula | Molecular Weight |
| Hydrous Dextrose USP | 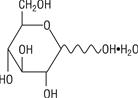 | 198.17 |
Not made with natural rubber latex, PVC or DEHP.
The plastic container is made from a multilayered film specifically developed for parenteral drugs. The solution contact layer is a rubberized copolymer of ethylene and propylene. The container is nontoxic and biologically inert. The container-solution unit is a closed system and is not dependent upon entry of external air during use. The container is overwrapped to provide protection from the physical environment and to provide an additional moisture barrier when necessary.