Dextrose
(Dextrose Monohydrate)Dextrose Prescribing Information
10% Dextrose Injection, USP (concentrated dextrose in water) in a partial-fill container is indicated for admixture with amino acids or dilution with other compatible IV fluids to provide a 5% final dextrose concentration for intravenous infusion in patients whose condition requires parenteral nutrition.
Concentrated Dextrose in Water is administered by slow intravenous infusion (a) after admixture with amino acid solutions or (b) after dilution with other compatible IV fluids. Dosage should be adjusted to meet the requirements of each individual patient.
The maximum rate at which dextrose can be infused without producing glycosuria is 0.5 g/kg of body weight /hr. About 95% of the dextrose is retained when infused at a rate of 0.8 g/kg/hr.
Clinical evaluation and periodic laboratory determinations are necessary to monitor changes in fluid balance, electrolyte concentrations, and acid-base balance during prolonged parenteral therapy or whenever the condition of the patient warrants such evaluation.
A list of nutritional admixture values is appended.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. (See
Electrolyte deficits, particularly in serum potassium and phosphate, may occur during prolonged use of concentrated dextrose solutions. Blood electrolyte monitoring is essential, and fluid and electrolyte imbalances should be corrected. Essential vitamins and minerals also should be provided as needed.
Solutions containing dextrose should be used with caution in patients with known subclinical or overt diabetes mellitus.
Care should be exercised to insure that the needle (or catheter) is well within the lumen of the vein and that extravasation does not occur.
Concentrated dextrose solutions should not be administered subcutaneously or intramuscularly.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
This product contains no more than 25 mcg/L of aluminum.
A concentrated dextrose solution should not be used when intracranial or intraspinal hemorrhage is present nor in the presence of delirium tremens if the patient is already dehydrated.
Dextrose Injection, USP without electrolytes should not be administered simultaneously with blood through the same infusion set because of the possibility that pseudoagglutination of red cells may occur.
Hyperosmolar syndrome, resulting from excessively rapid administration of concentrated dextrose may cause hypovolemia, dehydration, mental confusion and/or loss of consciousness.
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Some opacity of the plastic due to moisture absorption during sterilization process may be observed. This is normal and does not affect the solution quality or safety. The opacity will diminish gradually.
10% Dextrose Injection, USP (concentrated dextrose in water) is a sterile, nonpyrogenic, hypertonic solution of Dextrose, USP in water for injection for intravenous administration after appropriate admixture or dilution.
10% Dextrose Injection, USP is provided as a 500 mL volume in a 1000 mL partial-fill container. The container is designed to facilitate admixture or dilution.
See table under
10% Dextrose Injection, USP is supplied in single-dose, partial-fill, flexible containers as follows: a 500 mL volume in a 1000 mL container. See the following table.
NDC No. | % Conc. | Fill Volume (mL) | Total Grams of Dextrose Hydrous Per Container | kcal /100 mL (Per Container) | mOsmol/liter (calc.) | pH (range) |
0409-7938-19 | 10 | 500 | 50 | 34 (170) | 505 | 4.3 (3.2 to 6.5) |
| 0990-7938-19 | 10 | 500 | 50 | 34 (170) | 505 | 4.3 (3.2 to 6.5) |
ICU Medical is transitioning NDC codes from the "0409" to a "0990" labeler code. Both NDC codes are expected to be in the market for a period of time.
Dextrose Pre-Dilution Concentration | Admixture Non-Protein kcal/g N | Admixture Non-Protein kcal/liter | Admixture g N/liter | Admixture Dextrose Concentration |
10% | 31 | 170 | 5.5 | 5% |
Store at 20 to 25°C (68 to 77°F). [See USP Controlled Room Temperature.] Protect from freezing.
Revised: November, 2018
EN-4694
ICU Medical, Inc., Lake Forest, Illinois, 60045, USA
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only for use as a single-dose injection following admixture or dilution.
The flexible plastic container is fabricated from a specially formulated polyvinyl chloride. Water can permeate from inside the container into the overwrap but not in amounts sufficient to affect the solution significantly. Solutions in contact with the plastic container may leach out certain chemical components from the plastic in very small amounts; however, biological testing was supportive of the safety of the plastic container materials. Exposure to temperatures above 25°C/77°F during transport and storage will lead to minor losses in moisture content. Higher temperatures lead to greater losses. It is unlikely that these minor losses will lead to clinically significant changes within the expiration period.
Dextrose Injection, USP is a parenteral fluid and nutrient replenisher.
Dextrose Injection, USP is chemically designated D-glucose monohydrate (C6H12O6 • H2O), a hexose sugar freely soluble in water. It has the following structural formula:
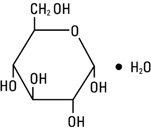
Water for Injection, USP is chemically designated H2O.