Diatrizoate Meglumine And Diatrizoate Sodium - Diatrizoate Meglumine And Diatrizoate Sodium solution
(Diatrizoate Meglumine And Diatrizoate Sodium)Diatrizoate Meglumine And Diatrizoate Sodium - Diatrizoate Meglumine And Diatrizoate Sodium solution Prescribing Information
Diatrizoate meglumine and diatrizoate sodium solution is indicated for radiographic examination of segments of the gastrointestinal tract (esophagus, stomach, proximal small intestine, and colon). The preparation is particularly indicated when a more viscous agent such as barium sulfate, which is not water-soluble, is not feasible or is potentially dangerous.
Diatrizoate meglumine and diatrizoate sodium solution may also be used as an adjunct to contrast enhancement in computed tomography of the torso (body imaging); the preparation is indicated, in conjunction with intravenous administration of a radiopaque contrast agent, when unenhanced imaging may not provide sufficient definition in distinguishing normal loops of bowel from adjacent organs or areas of suspected pathology.
This medium is not to be used for the preparation of solutions for parenteral administration. Oral or rectal administration only. Discard any unused portion after procedure.
The routine preparatory measures employed for barium studies are also appropriate for this agent.
For pediatric and severely cachectic patients the maintenance of an intravenous fluid line may be advisable.
For very young (under 10 kg) and debilitated children the dose should be diluted: 1 part diatrizoate meglumine and diatrizoate sodium solution in 3 parts water is recommended.
A usual adult dose is 240 mL of a dilute diatrizoate meglumine and diatrizoate sodium solution prepared by diluting 25 mL (9.17 g iodine) to one liter with tap water. Less dilute solutions [up to 77 mL (28.26 g iodine) diluted to one liter with tap water] may be used when indicated. The dose is administered orally about 15 to 30 minutes prior to imaging in order to permit the contrast medium to reach the pelvic loops.
Do not administer to patients with a known hypersensitivity to diatrizoate meglumine and diatrizoate sodium solution or any of its components.
Most adverse reactions to enteral diagnostic radiopaque agents are mild and transitory. Nausea, vomiting and/or diarrhea, urticaria with erythema, hypoxia, acute dyspnea, tachyarrhythmia, and anaphylaxis have occurred following ingestion of the contrast medium, particularly when high concentrations of large volumes of solution are administered. Severe changes in serum osmolarity and electrolyte concentrations may produce shock-like states (see
Diatrizoate Meglumine and Diatrizoate Sodium Solution, USP is a palatable strawberry-flavored water-soluble iodinated radiopaque contrast medium for oral or rectal administration only. Each mL contains 660 mg diatrizoate meglumine USP and 100 mg diatrizoate sodium USP; pH has been adjusted to 6.0 to 7.6 with sodium hydroxide.
Diatrizoate meglumine is designated chemically as 1-deoxy-1-(methylamino)-D-glucitol 3,5- diacetamido-2,4,6-triiodobenzoate (salt); diatrizoate sodium is monosodium 3,5-diacetamido-2,4,6-triiodobenzoate. Structural formulas:
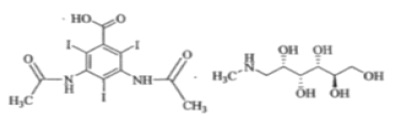
Diatrizoate meglumine C
11H
9I
3N
2O
4.C
7H
17NO
5
Molecular Weight: 809.13
Organically bound Iodine: 47.1%
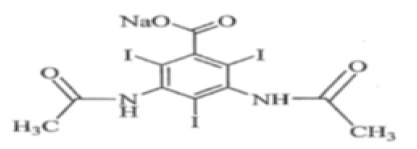
Diatrizoate sodium C
11H
8I
3N
2NaO
4
Molecular Weight: 635.90
Organically bound Iodine: 59.9%
The most important characteristic of contrast media is the iodine content. The relatively high atomic weight of iodine contributes sufficient radiodensity for radiographic contrast with surrounding tissues.
Diagnostic enteral radiopaque agents have few known pharmacological effects. Diatrizoate meglumine and diatrizoate sodium exert a mild laxative effect attributable to their high osmolarity.
Diatrizoate meglumine and diatrizoate sodium are sparingly absorbed from the intact gastrointestinal tract, and therefore permit gastrointestinal opacification and delineation after oral or rectal administration. Oral administration is used for radiographic evaluation of the esophagus, stomach and proximal small intestine. Rectal administration is used for examination of the colon; however, visualization of the distal small bowel is generally unsatisfactory, since the hypertonicity of the medium causes intraluminal diffusion of water with subsequent dilution of the medium. Enough absorption from the gastrointestinal tract to permit incidental visualization of the urinary tract has been reported; this should also be considered when thyroid testing is being contemplated, since iodine-mediated thyrotropic effects may occur (see