Diazoxide - Diazoxide suspension
(Diazoxide)Diazoxide - Diazoxide suspension Prescribing Information
Diazoxide oral suspension is indicated for the management of hypoglycemia due to hyperinsulinism associated with the following conditions:
- Adults: Inoperable islet cell adenoma or carcinoma, or extrapancreatic malignancy.
- Infants and children: Leucine sensitivity, islet cell hyperplasia, nesidioblastosis, extrapancreatic malignancy, islet cell adenoma, or adenomatosis. Diazoxide oral suspension may be used preoperatively as a temporary measure, and postoperatively, if hypoglycemia persists.
Diazoxide oral suspension should be used only after a diagnosis of hypoglycemia due to one of the above conditions has been definitely established. When other specific medical therapy or surgical management either has been unsuccessful or is not feasible, treatment with diazoxide oral suspension should be considered.
Patients should be under close clinical observation when treatment with diazoxide oral suspension is initiated. Carefully monitor the clinical response and blood glucose until the patient’s condition has stabilized satisfactory; in most instances, this may be accomplished in several days. If administration of diazoxide oral suspension is not effective after 2 or 3 weeks, discontinue diazoxide oral suspension.
Individualize the dosage of diazoxide oral suspension based on the severity of the hypoglycemic condition and the blood glucose level and clinical response of the patient. Adjust the dosage until the desired clinical and laboratory effects are produced with the least amount of diazoxide oral suspension. Take special care to ensure the accuracy of the dosage in infants and young children.
The recommended starting dosage is 3 mg/kg/day, administered orally, divided into 3 equal doses every 8 hours or 2 equal doses every 12 hours. The dosage may be titrated to a maximum of 8 mg/kg/day. Patients with refractory hypoglycemia may require higher dosages.
The recommended starting dosage is 8 mg/kg/day, administered orally, divided into 3 equal doses every 8 hours or 2 equal doses every 12 hours. The dosage may be titrated to a maximum of 15 mg/kg/day.
Diazoxide oral suspension is contraindicated in patients with:
- Functional hypoglycemia
- Hypersensitivity to diazoxide, any of the excipients in diazoxide oral suspension, or other thiazides
Sodium and fluid retention is most common in young infants and in adults and may precipitate congestive heart failure in patients with compromised cardiac reserve. (see
Diabetic ketoacidosis and hyperosmolar nonketotic coma may develop very rapidly. Monitor patients for up to 7 days due to the long half-life of diazoxide (see
Hirsutism of the lanugo type, mainly on the forehead, back and limbs, occurs most commonly in children and women and may be cosmetically unacceptable. It subsides on discontinuation of diazoxide oral suspension.
Hyperglycemia or glycosuria may require reduction in dosage in order to avoid progression to ketoacidosis or hyperosmolar coma.
Gastrointestinal intolerance may include anorexia, nausea, vomiting, abdominal pain, ileus, diarrhea, transient loss of taste.
Tachycardia, palpitations, increased levels of serum uric acid are common.
Thrombocytopenia with or without purpura may require discontinuation of diazoxide oral suspension. Neutropenia is transient, is not associated with increased susceptibility to infection, and ordinarily does not require discontinuation of diazoxide oral suspension. Skin rash, headache, weakness, and malaise may also occur.
There have been postmarketing reports of pericardial effusion in patients without structural heart disease; the majority of cases occurred in pediatric patients and infants.
Since diazoxide is highly bound to serum proteins, it may displace other substances which are also bound to protein, such as bilirubin or coumarin and its derivatives, resulting in higher blood levels of these substances. Concomitant administration of diazoxide oral suspension and diphenylhydantoin may result in a loss of seizure control. Consider these potential interactions when administering diazoxide oral suspension.
The concomitant administration of thiazides or other diuretics may potentiate the hyperglycemic and hyperuricemic effects of diazoxide.
Diazoxide Oral Suspension, USP is a nondiuretic benzothiadiazine derivative taken orally for the management of symptomatic hypoglycemia. Diazoxide oral suspension contains 50 mg of diazoxide, USP in each milliliter and has a chocolate-mint flavor; alcohol content is approximately 7.29%. Other ingredients include sorbitol solution, chocolate mint type flavor, propylene glycol, magnesium aluminum silicate, carboxymethylcellulose sodium, sodium benzoate, methylparaben, poloxamer 188, propylparaben, FD&C Red No. 40, FD&C Yellow No. 6 and purified water. Hydrochloric acid may be added to adjust pH.
Diazoxide has the following structural formula:
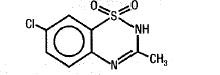
Diazoxide is 7-chloro-3-methyl-2H-1,2,4-benzothiadiazine 1,1-dioxide with the empirical formula C8H7ClN2O2S and the molecular weight 230.7. It is a white powder practically insoluble to sparingly soluble in water.